Abstract
Background: Attention-deficit/hyperactivity symptoms have repeatedly been associated with poor cognitive functioning. Genetic studies have demonstrated a shared etiology of attention-deficit/hyperactivity disorder (ADHD) and cognitive ability, suggesting a common underlying neurobiology of ADHD and cognition. Further, neuroimaging studies suggest that altered cortical development is related to ADHD. In a large population-based sample we investigated whether cortical morphology, as a potential neurobiological substrate, underlies the association between attention-deficit/hyperactivity symptoms and cognitive problems.
Methods: The sample consisted of school-aged children with data on attention-deficit/hyperactivity symptoms, cognitive functioning and structural imaging. First, we investigated the association between attention-deficit/hyperactivity symptoms and different domains of cognition. Next, we identified cortical correlates of attention-deficit/hyperactivity symptoms and related cognitive domains. Finally, we studied the role of cortical thickness and gyrification in the behaviour–cognition associations.
Results: We included 776 children in our analyses. We found that attention-deficit/hyperactivity symptoms were associated specifically with problems in attention and executive functioning (EF; b = −0.041, 95% confidence interval [CI] −0.07 to −0.01, p = 0.004). Cortical thickness and gyrification were associated with both attention-deficit/hyperactivity symptoms and EF in brain regions that have been previously implicated in ADHD. This partly explained the association between attention-deficit/hyperactivity symptoms and EF (bindirect = −0.008, bias-corrected 95% CI −0.018 to −0.001).
Limitations: The nature of our study did not allow us to draw inferences regarding temporal associations; longitudinal studies are needed for clarification.
Conclusion: In a large, population-based sample of children, we identified a shared cortical morphology underlying attention-deficit/hyperactivity symptoms and EF.
Introduction
Neurodevelopmental disorders have repeatedly been associated with poor cognitive functioning and lower levels of general intelligence in both clinical and epidemiological population-based samples.1,2 Studies of attention-deficit/hyperactivity disorder (ADHD) have shown moderate correlations between ADHD symptom scores and IQ scores and a significantly lower mean IQ in children with ADHD.3,4 Yet, it is unclear whether these deficits in cognitive functioning represent a general cognitive deficit, or whether they primarily reflect deficits in more specific cognitive domains. In order to parse out the specific cognitive problems, multiple studies have tested neuropsychological performance in clinical ADHD samples. These studies suggest that a wide range of neuropsychological domains is affected in patients with ADHD.3,5–7 Yet, 2 large meta-analyses demonstrated that ADHD seems most strongly associated with tasks assessing executive functioning (EF).3,6
Genetic studies have demonstrated a shared etiology of cognitive ability and child psychopathology in general and, more specifically, in children with ADHD.4,8 This shared genetic background suggests that a common neurobiology underlies ADHD and cognition. Previous neuroimaging studies in ADHD have shown a delay in brain maturation9 and a thinner cortex10 throughout most of the cerebrum. In population-based pediatric samples of both healthy children11 and the population at large12 the latter association has also been demonstrated. Studies of gyrification offer mixed results in children with ADHD. Some studies report a global decrease,13 while others report either a small, local increase14 or no abnormalities.15 Based on the shared genetic background of ADHD and cognition, one might expect that the shared neurobiology underlying ADHD and cognition could potentially be reflected in cortical morphology. However, to our knowledge, no studies have assessed the role of cortical morphology in the association between ADHD symptoms and cognitive functioning.
The notion that child psychopathology, such as ADHD, might be better described within a dimensional framework has recently gained support. Within this framework of continuous symptom levels, children with clinical disorders constitute the extreme end of the spectrum. Studies have suggested that symptoms of inattention and hyperactivity are part of a spectrum that extends into the general population and that a dimensional approach can further contribute to a better etiological understanding of child psychopathology.16–19 Also, for research purposes, the use of a continuous score provides more power and allows the application of advanced statistical methods. If ADHD symptoms are indeed part of a spectrum extending into the general population, one would also expect the neurobiological underpinnings of ADHD to lie on a continuum in the general population. However, the majority of studies to date exploring the neurobiology of ADHD symptoms and associated cognitive problems have been performed in clinical samples.
In the present large, population-based study of school-aged children, we investigated the role of cortical morphology in the association between ADHD symptoms and cognitive functioning. Based on previous studies, we selected 2 measures of cortical morphology that have been shown to be implicated in ADHD: cortical thickness and gyrification. These measures reflect brain morphological aspects that are regulated by different genetic mechanisms and tap different processes during development.20,21 We tested 2 hypotheses: first, that ADHD symptoms would be specifically associated with problems in EF and, second, that cortical morphology (the potential shared neurobiological substrate underlying both ADHD and cognitive problems) could partly explain this association.
Methods
Participants
This study is embedded in the Generation R Study, a population-based cohort study in Rotterdam, the Netherlands.22 When their children were around the age of 6 years, the parents of 6346 children reported on their childrens’ behaviour. In the same period, a brain MRI study began within a subsample of the study. Between September 2009 and July 2013, a total of 1325 children were recruited.23
As a part of the present neuroimaging study, we performed an extensive neuropsychological assessment. We included children who had structural imaging data of good quality available. We excluded children who were missing Child Behavior Checklist (CBCL) attention-deficit/hyperactivity problems scale data. Exclusion based on image quality was not related to the CBCL attention-deficit/hyperactivity problems score. We also excluded twins and a randomly selected child from each sibling pair. Finally, since attention-deficit/hyperactivity symptoms are known to be comorbid with autism traits, all children with a score above the screening cutoff for autism traits on the Social Responsiveness Scale24 were excluded from the analyses. The Medical Ethics Committee of the Erasmus Medical Center approved our study protocol, and we obtained written informed consent from the parents of all participants.
Attention-deficit/hyperactivity symptoms
Attention-deficit/hyperactivity symptoms were measured using the DSM-oriented attention-deficit/hyperactivity problems scale score of the CBCL 1.5–5 at 6 years of age.25 All children were assessed using a single instrument; the preschool CBCL was chosen because many children were younger than 6 years, and older-age versions are inappropriate for such young children. In the CBCL 1.5–5, the primary caregiver is asked to answer 99 questions on a 3-point scale regarding the behaviour of their child; 6 of the items make up the attention-deficit/hyperactivity problems scale. Good reliability and validity have been reported.25 The Cronbach α was similar between the 5-year-old children and children aged 6 years and older (α = 0.80 and α = 0.83, respectively), indicating that the attention-deficit/hyperactivity symptoms were reliably measured in the children older than 5 years.
Cognitive functioning
Cognitive functioning was assessed as a part of the neuroimaging study in children aged 6–9 years using a shortened version of the Developmental Neuropsychological Assessment (NEPSY-II-NL).26 The NEPSY-II-NL is a Dutch translation of the North-American NEPSY-II and assesses neuropsychological functioning in children aged 5–12 years, covering different domains of neuropsychological functioning, including attention and EF, language, memory and learning, sensorimotor functioning and visuospatial processing.27
In order to limit multiple testing, we analyzed summary domain scores. Since the NEPSY-II-NL does not provide domain scores, we used the first unrotated factor score of the principal components analyses that we performed on the test scores comprising each predefined NEPSY-II-NL domain in all children in the brain imaging study who had NEPSY-II-NL data available (n = 1307; Appendix 1, Table S1, available at jpn.ca). As different summary scores in the sensorimotor domain may reflect distinct strategies (e.g., fast with many errors v. slow but more accurate), it was not possible to derive a single meaningful sensorimotor component out of the separate scores. Therefore, using a different approach, 2 separate scores were derived. The primary sensorimotor score is a speed–accuracy trade-off score, generated by computing the standardized product of the completion time and errors, while the secondary sensorimotor score is a standardized score for the number of compensatory pencil lifts while performing the task.
Cortical morphology
We acquired MRIs with a GE Discovery MR750 3.0 T scanner (GE Healthcare Worldwide) using an 8-channel head coil. A high-resolution T1-weighted image was collected using an inversion recovery prepared fast spoiled gradient recalled-echo (IR-FSPGR) sequence with the following parameters: repetition time (TR) 10.3 ms, echo time (TE) 4.2 ms, inversion time (TI) 350 ms, number of excitations = 1, flip angle 16°, matrix 256 × 256, imaging acceleration factor of 2, and an isotropic resolution of 0.9 × 0.9 × 0.9 mm3. We performed cortical reconstruction using the FreeSurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/) version 5.1.28 Image quality assurance was performed in 2 steps: first, with a visual inspection of the image quality of the T1 sequence before preprocessing the data and, second, with a visual inspection of the surface reconstruction quality after the images were processed through the FreeSurfer pipeline. Both steps of quality control had to be passed successfully in order for data to be included in the analyses.29 Neuroimaging measures of interest were cortical thickness and gyrification. We calculated cortical thickness as the closest distance from the grey/white matter boundary to the grey matter/cerbrospinal fluid (CSF) boundary at each vertex on the tessellated surface.30 The surface-based thickness maps were smoothed using a 10 mm full-width at half-maximum (FWHM) Gaussian kernel before the analyses. To assess gyrification we used the local gyrification index (lGI)31 implemented in FreeSurfer. This approach provides an estimation of the local gyrification index, taking into account the 3-dimensional cortical surface. Identification of the pial and white surfaces against an additional surface that tightly wraps the pial surface was used to estimate the degree of cortical folding at a 25 mm spherical vertex-based region. The surface-based lGI maps were smoothed using a 5 mm FWHM Gaussian kernel before the analyses.
Covariates
We considered the children to be Dutch if both parents were born in the Netherlands and non-Dutch if 1 or both parents were born elsewhere.32 Maternal education was defined as the highest level of education completed,33 and household income was defined as total net monthly income. We obtained information on maternal alcohol use and smoking during pregnancy using questionnaires, and information on date of birth, sex and birth weight was obtained from midwives and hospital registries. Gestational age was established using ultrasound measures during pregnancy. Nonverbal IQ of the child was assessed at around age 6 years using a shortened version of the Snijders-Oomen Niet-verbale Intelligentie Test–Revisie (SON-R 2.5–7).34 The use of psychostimulant medication was recorded during the MRI visit. All these covariates were selected based on their relevance according to the literature and added to our analyses.
Statistical analysis
Missing values of potential confounding factors (child nationality 0.05%, IQ 16.6%, psychostimulant medication use 3.0%, household income 8.3%, maternal education 2.9%, drinking during pregnancy 15.8%, and smoking during pregnancy 11.1%) were imputed using the multiple imputation (MCMC) method, with 5 imputations and 10 iterations. For 13 children, the local gyrification software failed to run, and indices within the identified clusters could not be extracted. Therefore, these were imputed using the folding index, mean curvature, Gaussian curvature, intrinsic curvature and global hemispheric gyrification index measures as estimates, which are equivalent to or highly correlated with the local gyrification index. Excluding these 13 children did not change results. The CBCL attention-deficit/hyperactivity problems score and all NEPSY-II-NL scores were complete in all selected children. The CBCL attention-deficit/hyperactivity problems score and the NEPSY-II-NL overall score, attention and EF domain score and the visuospatial processing domain score were square root–transformed to approach a normal distribution. In all SPSS analyses, we controlled for the effect of age by residualizing the CBCL score, the NEPSY domain scores, and the extracted cortical clusters for age. We added other potential confounders to the linear regression analyses as covariates.
The analyses were performed in 3 steps, as described below.
Cognitive functioning
First, to test our hypothesis that associations between ADHD symptoms and cognitive functioning would be specific to problems in EF, we performed linear regression analyses testing the association between the CBCL attention-deficit/hyperactivity problems score and the NEPSY-II-NL overall and domain scores. Furthermore, we assessed the association between ADHD symptoms and IQ using regression analyses.
Cortical morphology
In order to be able to test our second hypothesis — that cortical morphology would explain the association between attention-deficit/hyperactivity symptoms and cognitive functioning — we studied the direct association between CBCL attention-deficit/hyperactivity problems and cortical morphology as well as the direct association between NEPSY-II-NL cognitive functioning and cortical morphology (only for cognitive domain(s) that were associated with attention-deficit/hyperactivity symptoms). We performed whole-brain vertex-wise general linear model analyses using the FreeSurfer built-in module QDEC. Age during scanning and sex were included as covariates. To correct for multiple testing of all brain vertices, we performed Monte Carlo null-Z simulations using a threshold of 1.3 (p < 0.05), controlling the rate of false-positive clusters. Following the vertex-wise analyses in QDEC, we extracted the cortical thickness/gyrification data of significant cluster(s) for each individual and imported these into SPSS to test whether the association was potentially confounded by other factors by performing cluster-wise regression analyses with additional covariates.
Interrelation between cognitive functioning, cortical morphology and ADHD symptoms
Next, we tested our second hypothesis — that cortical morphology is a shared neurobiological substrate underlying both ADHD and cognitive problems, and would thus explain at least part of the association between attention-deficit/hyperactivity symptoms and cognition. To this aim, we performed a multiple mediation analysis (in which all mediators were entered in the model at once) of the association between CBCL attention-deficit/hyperactivity problems with NEPSY-II-NL cognitive functioning by cortical morphology. The mediation analysis was performed using the PROCESS macro in SPSS (www.afhayes.com/) with bias-corrected bootstrapping using 1000 bootstrap samples.35 We chose a mediation analysis as a statistical method because it allows us to assess cortical morphology as a potential shared biological substrate of attention-deficit/hyperactivity symptoms and cognitive functioning. However, we made no assumptions about directionality in these associations.
We selected those cortical clusters as potential mediators that were associated with both attention-deficit/hyperactivity symptoms and cognitive functioning. To this aim, we performed retention/consistency analyses; clusters that were detected using the CBCL attention-deficit/hyperactivity problems score were tested for their association with the NEPSY-II-NL score(s) and vice versa. Clusters were retained and added as a mediator in the respective mediation analysis only if they also showed a significant or trend-level (p < 0.1) association with the other measure. This threshold of consistency was deliberately set to be more lenient, because an overall mediation effect can be significant even if the separate building blocks are not all as strongly associated.36
Results
Study sample
The neuropsychological assessment in the neuroimaging study was performed in 1307 children. Of these, 1053 children also had structural imaging data available, and after quality control, 907 children remained. After excluding children missing CBCL attention-deficit/hyperactivity problems scale data (n = 82), twins (n = 17), a randomly selected child from each sibling pair (n = 11) and children with a score above the screening cut-off for autism traits on the Social Responsiveness Scale (n = 21), we were left with a final study sample of 776 children. The demographic and clinical characteristics of study participants are presented in Table 1.
Demographic and clinical characteristics of the study sample
Cognitive functioning
Attention-deficit/hyperactivity symptoms were strongly related to IQ (β = −0.16, p < 0.001). After correction for the covariates (child sex, nationality, gestational age at birth, birth weight, psychostimulant use, maternal education, drinking during pregnancy, smoking during pregnancy and household income) this association remained (β = −0.09, p = 0.022).
The analyses for the separate cognitive domains showed that after adjustment for all covariates mentioned above plus IQ, attention-deficit/hyperactivity symptoms were associated with functioning in only 1 cognitive domain: attention and EF (β = −0.11, p = 0.004). Children with more symptoms performed significantly worse on the tasks comprising this domain. No significant associations were found for any of the other cognitive domains (Appendix 1, Table S2).
Cortical morphology
Detection of cortical clusters
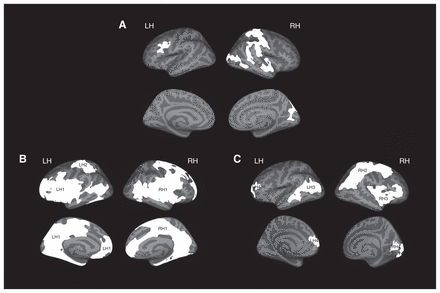
Figure 1, Table 2 and Appendix 1, Table S3, show the association between attention-deficit/hyperactivity symptoms and cortical thickness. We detected 5 clusters in which more attention-deficit/hyperactivity symptoms were associated with a thinner cortex. The first cluster was located in the left caudal middle frontal gyrus, encompassing parts of the rostral middle frontal gyrus (β = −0.14, p < 0.001). The second cluster was a large cluster in the right postcentral gyrus, spreading toward the precentral gyrus and the superior parietal, superior temporal and middle temporal gyri (β = −0.22, p < 0.001). The third cluster was localized in the right lateral occipital gyrus, spreading toward the inferior temporal gyrus (β = −0.19, p < 0.001). The fourth cluster consisted of the right superior temporal gyrus (β = −0.16, p < 0.001), and the fifth was localized in the right occipital gyrus (β = −0.15, p < 0.001).
Significant clusters of vertex-wise associations between (A) Child Behavior Checklist (CBCL) attention-deficit/hyperactivity problems scores and cortical thickness, (B) CBCL attention-deficit/hyperactivity problems scores and local gyrification index and (C) Developmental Neuropsychological Assessment (NEPSY-II-NL) attention and executive functioning scores and local gyrification index. Clusters indicate regions of a thinner cortex/less gyrification in relation to more symptoms/worse functioning. LH = left hemisphere; RH = right hemisphere.
Cluster-wise regression analyses of the association between CBCL ADHP score, NEPSY-II-NL ATT/EF score and cortical thickness*
Vertex-wise cortical analysis did not show an association between cortical thickness and performance in the NEPSY-II-NL domain attention and EF after correction for multiple testing.
Figure 1, Table 3 and Appendix 1, Table S3, show the association between attention-deficit/hyperactivity symptoms and gyrification. We detected 3 large clusters in which more attention-deficit/hyperactivity symptoms were related to less gyrification. Because the clusters were large and comprised different lobes and both the lateral and medial regions of the brain, we provide a global label for each cluster. The first left hemisphere cluster (LH1) was a large cluster, covering parts of the frontal, temporal and parietal regions of the brain (β = −0.14, p < 0.001). The second cluster (LH2) was localized to the left superior parietal/postcentral gyrus (β = −0.11, p = 0.006). The right hemisphere cluster (RH1) covered the frontal, temporal and parietal areas of the brain (β = −0.13, p = 0.001).
Cluster-wise regression analyses of the association between CBCL ADHP score, NEPSY-II-NL ATT/EF score and gyrification*
Figure 1, Table 3 and Appendix 1, Table S3, also show the association between NEPSY-II-NL attention and EF and gyrification. We detected 5 clusters in which worse functioning on the NEPSY-II-NL attention and EF domain was related to a lower local gyrification index. The first cluster (LH3) was located in the left inferior parietal area (β = 0.08, p = 0.030). The second cluster (LH4) covered a part of the left frontal area (β = 0.09, p = 0.020). The first cluster in the right hemisphere (RH2) was a large cluster covering the parietal lobe and extending into the frontal lobe (β = 0.11, p = 0.004). The second right hemisphere cluster (RH3) covered parts of frontal and temporal areas (β = 0.07, p = 0.07). Another cluster (RH4) was located in the right occipital lobe (β = 0.08, p = 0.023).
We performed supplementary sex-stratified analyses assessing potential differences between boys and girls (Appendix 1, Tables S4 and S5). These supplementary analyses showed that the behavioural phenotype and cortical thickness cluster associations were similar in boys and girls. Sex-stratified results for gyrification were also similar in boys and girls for clusters found to be associated with the CBCL attention-deficit/hyperactivity problems score. Results for gyrification clusters found to be associated with the NEPSY-II-NL attention and EF score were stronger in girls than boys. Additional analyses assessing sex interaction effects for these NEPSY-II-NL attention and EF–gyrification associations showed a significant sex interaction effect only for the right occipital lobe gyrification (RH4) cluster. In this cluster the effect was primarily present in girls. The regression lines for boys and girls for this cluster are shown in Appendix 1, Figure S1.
Finally, to assess whether the brain–behaviour associations were visible across the entire spectrum of ADHD symptoms and to make sure associations were not solely driven by extreme clinical ADHD cases, supplementary analyses were performed. In these analyses, children scoring above the clinical cut-off of the CBCL attention-deficit/hyperactivity problems scale (n = 32) were excluded.25 Results of the analyses were similar (Appendix 1, Tables S6 and S7).
Retention of cortical clusters
Because an association with both the behavioural and cognitive measures was a prerequisite for a cluster to be selected for the mediation analysis, we subsequently tested whether clusters detected with either of the 2 measures were also related to the other measure. The results of these retention analyses are shown in Table 2 and Table 3. Four identified clusters were retained and added to the mediation analyses.
Interrelation between cognitive functioning, cortical morphology and ADHD symptoms
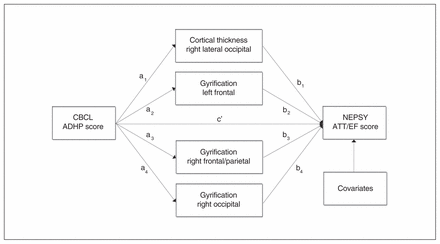
Finally, we investigated whether the association between attention-deficit/hyperactivity symptoms and cognitive functioning in the domain attention and EF could be explained by the cortical morphology of the identified clusters. To this aim we performed a multiple mediation analysis, using as mediators only those clusters that were retained in the previous step. All selected cortical clusters were entered at once in the same model. The multiple mediation model is depicted in Figure 2, estimating the total indirect effect of the CBCL attention-deficit/hyperactivity problems score on the NEPSY attention and EF score through the selected cortical clusters (M1 = a1b1, …. M4 = a4b4) and the direct effect of the CBCL attention-deficit/hyperactivity problems score on the NEPSY attention and EF score (c′).
Multiple mediation model estimating the total indirect effect of the Child Behavior Checklist (CBCL) attention-deficit/hyperactivity problems (ADHP) score on the Developmental Neuropsychological Assessment (NEPSY) attention and executive functioning (ATT/EF) score through the selected cortical clusters (M1 = a1b1, …. M4 = a4b4) and the direct effect of the CBCL ADHP score on the NEPSY attention and EF score (c′).
The mediation analysis showed that both the direct effect of CBCL attention-deficit/hyperactivity problems on NEPSY-II-NL attention and EF (b = −0.033, p = 0.020) and the total indirect effect through the selected cortical clusters (b = −0.008, bias-corrected 95% confidence interval [CI] −0.018 to −0.001) were statistically significant. This implies that the association between attention-deficit/hyperactivity symptoms and cognitive functioning in the domain attention and EF (b = −0.041, p = 0.004) was at least partially explained by cortical morphology. The ratio of the indirect effect to the total effect (PM), or relative indirect effect, was 0.19 (95% CI 0.01–0.74), suggesting that the cortical clusters explain about one-fifth of the total association between CBCL attention-deficit/hyperactivity problems and NEPSY-II-NL attention and EF. The ratio of the indirect effect to the direct effect (RM) was 0.23 (95% CI 0.01–1.74), indicating that the indirect effect is approximately 0.23 times the size of the direct effect.
When analyses were repeated with only the 2 significant clusters associated with both the CBCL attention-deficit/hyperactivity problems and NEPSY-II-NL attention and EF (LH and RH4) as mediators, the results remained identical.
Discussion
The aim of this study was to investigate the role of cortical thickness and gyrification in the association between attention-deficit/hyperactivity symptoms and cognitive functioning in the general population. As hypothesized, we found that attention-deficit/hyperactivity symptoms were not associated with cognitive functioning in general, but specifically to functioning in the domain of attention and EF. This finding is in line with those of previous clinical studies that also showed deficits in EF to be most strongly associated with ADHD.3,6 These findings are consistent with one of the most influential theories of ADHD, suggesting that the symptoms and cognitive problems in individuals with ADHD actually result from a core deficit in inhibition.37 It is possible that the weaker general cognitive functioning in previous ADHD studies3,4 was partly driven by deficits in EF. Since the tasks in our study were designed to measure specific cognitive functions, with minimized interference of other functions, the specificity of EF problems in attention-deficit/hyperactivity problems could be tested. Another potential explanation could be that these previous studies were performed in clinical populations, in whom symptoms are usually more severe and in whom there is a greater chance of referral bias. In line with the results of previous clinical studies,38 we found attention-deficit/hyperactivity symptoms to be associated with a thinner cortex over all 4 lobes of the brain. Similarly, we found less gyrification throughout large areas of the brain. Finally, we found similarly located clusters of less gyrification in children who performed worse on neuropsychological tasks measuring attention and EF.
Because of the shared genetic etiology of cognitive ability and ADHD,4,8 which suggests a common underlying neurobiology of attention-deficit/hyperactivity symptoms and cognition, and based on previous neuroimaging findings in ADHD9,10,13,14 we hypothesized that cortical morphology (as reflected by cortical thickness and gyrification) could be the shared substrate underlying attention-deficit/hyperactivity symptoms and cognitive problems. Our results show that a shared cortical morphology partly explained the association between attention-deficit/hyperactivity symptoms and EF. This finding enhances our understanding of the underlying neurobiology of attention-deficit/hyperactivity symptoms and co-occurring EF problems. Potentially, future studies investigating other imaging modalities (e.g., connectivity) may provide additional knowledge with regard to the shared neurobiology underlying these 2 constructs. Based on our results, it can be concluded that attention-deficit/hyperactivity and EF problems are at least partly explained by similar cortical abnormalities in thickness and gyrification, indicating that the EF problems in children with ADHD should not be seen as a completely separate comorbid cognitive problem, but as part of the disorder. The cortical abnormalities that we found were nonspecific and covered large parts of the cortex, which suggests that both attention-deficit/hyperactivity problems and problems in EF are related to global deviations in cortical morphology. This finding is in line with those of previous clinical studies that have shown widespread cortical abnormalities.9,10,13
An important strength of the present study is that, to our knowledge, no previous studies have assessed the role of cortical thickness and gyrification in the association between attention-deficit/hyperactivity symptoms and cognitive functioning. Studying this topic helps us to understand the underlying neurobiology and high comorbidity of attention-deficit/hyperactivity symptoms and cognitive problems. In addition, the association between gyrification and attention-deficit/hyperactivity symptoms has never been tested in a nonclinical population. The population-based nature, as well as the large sample size, are other important strengths of our study. By using a continuous problem score, our study covers the entire spectrum of attention-deficit/hyperactivity symptoms and thus includes both children with no or very few symptoms as well as children with clinical symptoms. This provides greater generalizability with the general population compared with a study sample recruited from a clinical setting. Furthermore, we were able to correct for many confounding factors, including the use of psychostimulant medication. As psychostimulant use may alter brain structure38 and influences cognitive functioning,39,40 this is a very important potentially confounding factor to take into account. Although our ability to correct for many confounding factors may be considered a strength, it may have also led to some overcorrecting and attenuation of effects. However, fully adjusted results did not differ substantially from unadjusted results.
Limitations
A limitation of our study is that the neuroimaging and neuropsychological data were collected at only 1 time point, therefore no inferences on causality or direction of effect can be made. Although we chose to perform a mediation analysis to formally test the role of cortical morphology in the association between attention-deficit/hyperactivity symptoms and EF, we did not assume a causal pathway, and our study does not draw any conclusions about the directionality in the associations studied. This crucial information, whether behavioural problems precede cognitive problems or vice versa, and how exactly cortical thickness and gyrification are involved, remains to be elucidated. Longitudinal studies are needed to clarify this temporal direction. Also, the CBCL data were collected at a slightly earlier time point than the neuroimaging and neuropsychological data (mean time interval 1.9 yr) and, although the CBCL attention-deficit/hyperactivity problems scores have been shown to have high stability over time in both clinical and population-based samples,41,42 this may have influenced the results. However, that associations remain despite a lag between measurements suggests a highly robust finding. Finally, since our measure of EF was a global measure, it would be interesting for future research to investigate whether specific aspects of EF (e.g., inhibition, working memory, planning, switching) are similarly related to ADHD symptoms and brain morphology.
Conclusion
In a large population-based sample of school-aged children we found cortical thickness and gyrification to be related to attention-deficit/hyperactivity symptoms and EF and to partly explain the association between these 2 constructs. This suggests that cortical morphology is a shared neurobiological substrate underlying attention-deficit/hyperactivity symptoms and EF.
Acknowledgements
The Generation R Study is conducted by the Erasmus Medical Center in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, Rotterdam, the Rotterdam Homecare Foundation, Rotterdam and the Stichting Trombosedienst & Artsenlaboratorium Rijnmond (STAR-MDC), Rotterdam. We gratefully acknowledge the contribution of children and parents, general practitioners, hospitals, midwives and pharmacies in Rotterdam. The general design of Generation R Study is made possible by financial support from the Erasmus Medical Center, Rotterdam, the Erasmus University Rotterdam, the Netherlands Organization for Health Research and Development (ZonMw), the Netherlands Organisation for Scientific Research (NWO), the Ministry of Health, Welfare and Sport and the Ministry of Youth and Families. In addition, this study is financially supported through NWO ZonMw (TOP project number 91211021); and the NWO Brain & Cognition (project number 433-09-228).
Footnotes
Competing interests: F.C. Verhulst is head of the Department of Child and Adolescent Psychiatry/Psychology at the Erasmus Medical Center, which publishes the Achenbach System of Empirically Based Assessment (ASEBA) and from which he receives remuneration. No other competing interests declared.
Contributors: S. Mous, T. White and H. Tiemeier designed the study. S. Mous, T. White, R. Muetzel, H. El Marroun, V. Jaddoe, F. Verhulst, D. Posthuma and H. Tiemeier acquired the data, which S. Mous, T. White, J. Rijlaarsdam, T. Polderman and H. Tiemeier analyzed. S. Mous wrote the article, which all authors reviewed and approved for publication.
- Received December 9, 2015.
- Revision received March 22, 2016.
- Accepted April 27, 2016.