Abstract
Background: Several clinical and radiological markers of early neurodevelopmental deviations have been independently associated with cognitive impairment in patients with schizophrenia. The aim of our study was to test the cumulative and/or interactive effects of these early neurodevelopmental factors on cognitive control (CC) deficit, a core feature of schizophrenia.
Methods: We recruited patients with first-episode schizophrenia-spectrum disorders, who underwent structural MRI. We evaluated CC efficiency using the Trail Making Test (TMT). Several markers of early brain development were measured: neurological soft signs (NSS), handedness, sulcal pattern of the anterior cingulate cortex (ACC) and ventricle enlargement.
Results: We included 41 patients with schizophrenia in our analysis, which revealed a main effect of ACC morphology (p = 0.041) as well as interactions between NSS and ACC morphology (p = 0.005), between NSS and handedness (p = 0.044) and between ACC morphology and cerebrospinal fluid (CSF) volume (p = 0.005) on CC measured using the TMT-B score – the TMT-A score.
Limitations: No 3- or 4-way interactions were detected between the 4 neurodevelopmental factors. The sample size was clearly adapted to detect main effects and 2-way interactions, but may have limited the statistical power to investigate higher-order interactions. The effects of treatment and illness duration were limited as the study design involved only patients with first-episode psychosis.
Conclusion: To our knowledge, our study provides the first evidence of cumulative and interactive effects of different neurodevelopmental markers on CC efficiency in patients with schizophrenia. Such findings, in line with the neurodevelopmental model of schizophrenia, support the notion that CC impairments in patients with schizophrenia may be the final common pathway of several early neurodevelopmental mechanisms.
Introduction
There is a large consensus that neurocognitive deficits, particularly executive dysfunction, are core features of patients with schizophrenia.1,2 These cognitive deficits are associated with treatment-refractory negative symptoms and poor functional outcomes.3 Elucidating more clearly the pathophysiology and pathogenesis of executive deficits in patients with schizophrenia is a first step to successfully optimizing cognitive remediation programs to treat these deficits and greatly improve the quality of life of patients with the disorder.2
Impaired executive functioning has been consistently reported in patients with schizophrenia compared with controls.1 In their meta-analysis of 204 studies, Heinrichs and Zakzanis4 reported that patients with schizophrenia are characterized by a broadly cognitive impairment in all ability domains, covered by 22 neurocognitive tests. Yet, the question of whether those differences reflect altered neurodevelopmental processes, a neurodegenerative course or medication effects is still debated as samples have mostly included middle-aged and predominantly chronic schizophrenia samples.3 To address this issue, Mesholam-Gately and colleagues3 performed a new meta-analysis focusing on patients with first-episode schizophrenia and showed that impairments were already present during the first episode approaching or at the same level as that found in patients with well-established illness. This suggests an early deterioration between premorbid and first-episode phases, followed by a relative stability at the group level.5 These findings showed how crucial the period before the illness onset is, with probable common neural pathways due to altered neurodevelopmental processes leading to symptom onset and cognitive deterioration.
Several lines of evidence support that executive function impairments are consequences of early developmental processes and are present during childhood premorbid stages. Birth cohort studies consistently report strong associations between poor performance on executive batteries during childhood and subsequent increased risk for schizophrenia.5 Prospective studies of young relatives at risk for schizophrenia also found that participants in whom the disorder later developed were more likely to present executive deficits during childhood,6,7 suggesting that executive dysfunction in patients with schizophrenia may trace back to childhood or earlier.6 Furthermore, recent studies support the hypothesis that cognitive dysfunction may be associated with deviations during early stages of brain development, including pre- and perinatal periods. Serologically documented infections in utero were found to be associated with subsequent impaired performance in the executive domain in patients with schizophrenia.8 An association between lower birth weight, a global proxy measure of uterine “optimality,”9 and neurocognitive deficits has also been found in patients with schizophrenia-spectrum disorders compared with controls.10
In this context, the aim of our study was to investigate the influences of early brain deviation on executive deficit in patients with schizophrenia. We considered different clinical and radiological markers of early brain development that were reportedly associated with schizophrenia liability and cognitive impairments: neurological soft signs (NSS),11 mixed handedness,12 ventricle enlargement13 and cortex morphology (sulcation).14 All of these factors were previously investigated independently, but to our knowledge no study to date has tested their possible combination effect, including cumulative effects and interactive effects.
Methods
Participants
We recruited participants from the Sainte-Anne First Episode study15 aged 18–45 years who had psychotic symptoms reaching “psychosis threshold” for the first time and who were freely admitted to hospital. Psychosis threshold was defined as a total score greater than 50 on the 24-item Brief Psychiatric Rating Scale, including a hallucinatory behaviour score greater than 3, a conceptual disorganization score greater than 4, an unusual thought content score greater than 4 and an emotional withdrawal score greater than 4.15 Exclusion criteria were any previous treated or untreated psychotic episode, serious medical and neurologic disorders, head injury, mental retardation, substance dependence or abuse during the previous year and inability to give consent for any treatment (forced hospitalization).
Patients were examined in the acute psychotic state directly or shortly after admission to hospital. The time between intake/consent into the study and MRI scanning and clinical/cognitive evaluation was less than 3 days.
The procedures of the study fulfilled the recommendations of the Declaration of Helsinki and followed French ethical regulations. This study was approved by the local ethical committee (CPP Ile-de-France III, France). Patients received a detailed information form, and further information was given during a face-to-face interview with a clinical investigator. Patients were allowed 24 h for reflection before signing the consent form, and a copy of the consent form was given to all patients. In addition, we confirmed their agreement during the follow-up and before discharge. None of the patients asked to withdraw his/her consent.
For the present study, only participants who had completed psychological assessments and MRIs were included.
Clinical assessment
Each patient underwent a clinical evaluation using a structured lifetime psychiatric interview (DIGS 3.0) and a psychopathology assessment using the Positive and Negative Syndrome Scale (PANSS). Patients were included in the study at their first consultation or admission, and were periodically reassessed in a longitudinal design at 6–24 months, so that final DSM-IV diagnoses were given with respect to duration criteria.
Cognitive assessment
We assessed executive efficiency using the Trail Making Test (TMT), which requires both flexibility, inhibition and working memory.16 The TMT has 2 parts: in part A the participant is required to draw lines sequentially connecting 23 numbers, and in part B the participant is required to draw lines sequentially connecting 13 numbers and 12 letters distributed on a sheet of paper. Numbers and letters are encircled and must be connected alternatively (e.g., 1, 2, 3, etc. in part A; 1, A, 2, B, 3, C, etc. in part B). The TMT-A and TMT-B scores correspond to the total time required to complete the tasks. Cognitive control (CC) efficiency was then derived from the difference between TMT-B and TMT-A scores.17 The TMT captures attention and processing speed as well as executive ability in cognitive switching when part B is administered after part A.18 Hence, executive elements of cognitive control can be isolated from attention and processing speed components using the differential score TMT-B – TMT-A.17 Longitudinal assessment was conducted for 18 patients who were reassessed when they were stabilized after 6 months (n = 12), 12 months (n = 2), 24 months (n = 1), or 36 months (n = 3).
MRI acquisition and preprocessing
Individual high-resolution anatomic inversion recovery T1-weighted images (3D spoiled gradient recalled [SPGR]) were acquired on a 1.5 T GE scanner using the following parameters: repetition time (TR) 10.3 ms, inversion time (TI) 450 ms, echo time (TE) 2.2 ms, flip angle 15°, bandwidth 11.9 kHz, 142 axial sections of 1.2 mm thickness, field of view (FOV) 240 × 240 mm2, voxel size 0.93 × 0.93 × 1.2 mm2, matrix 256 × 256, acquisition time 6 min 58 s. These MRIs were adapted to the reconstruction of the fine individual cortical folds.19
An automated preprocessing step skull-stripped T1-weighted MRIs and segmented the brain tissues. No spatial normalization was applied to MRIs to overcome potential bias that may result from the sulcus shape deformations induced by the warping process. The cortical folds were automatically segmented throughout the cortex from the skeleton of the grey matter/cerebrospinal fluid (CSF) mask, with the cortical folds corresponding to the crevasse bottoms of the “landscape,” the altitude of which is defined by its intensity on the MRIs. This definition provides a stable and robust sulcal surface definition that is not affected by variations in cortical thickness or grey matter/white matter contrast.20 For each participant, images at each processing step were visually checked. No gross segmentation error (e.g., cortical ribbon thinning, gyrus or sulcus missing) was detected. Image analysis was performed using the Morphologist toolbox in BrainVISA 4.0 software (http://brainvisa.info).
Markers of early brain development
Neurological soft signs
We administered comprehensive standardized neurological examinations,21 including the evaluation of 23 NSS (4-level rating), to each patient. This scale encompasses 3 main factors:21 sensory integration (face-hand test, graphesthesia, constructive apraxia, stereognosia, right/left recognition), motor integration (balance, romberg, finger to nose, gait) and motor coordination (rapid alternative movements of foot, hand, finger opposition, foot and hand dysrythmia, fist edge palm). Inter-rater reliability was 0.82 for the whole neurologic evaluation, and had a Chronbach α of 0.85 for internal consistency.21 The presence of NSS was defined from the normal distribution of total NSS among healthy individuals: patients were considered to have significant NSS (NSS participant) if their total NSS score was greater than 10, as 95% of healthy individuals have a total NSS score between 0 and 10. Patients with a total NSS score between 0 and 10 were therefore considered to have nonsignificant NSS (no NSS participant).
As handedness was also independently investigated (see the next section), the classification of participants as “NSS” or “no NSS” was determined after removing the laterality (#20) item from the scale.21 Notably, removing the laterality item did not affect the NSS versus no NSS group attribution.
Handedness
We assessed handedness using the Edinburgh Handedness Inventory (EHI). Participants were asked to demonstrate their performance for 10 hand-based tasks (using the words, “show me how you [...]”). A laterality quotient (LQ) was calculated as follows: LQ = [(R − L) ÷ (R + L)] × 100, where R is the number of right-hand tasks and L is the number of left-hand tasks). The LQ lies within a continuum of −100 (completely left-handed) to +100 (completely right-handed). Participants were group-coded according to previously defined cut-offs23: they were considered to be left-handed if the LQ was −100 to −71, mixed-handed (i.e., neither strong right- nor strong left-handed preference) if the LQ was −70 to +70 and right-handed if the LQ was +71 to +100.
Anterior cingulate cortex morphology
The sulcal pattern of the ACC was visually assessed using 3D mesh-based reconstruction of cortical folds to measure the occurrence and extent of local sulci.24 This 3D approach was used to overcome methodological issues inherent to the analysis of the sulcal pattern of the ACC from the 2D sagittal slices.24
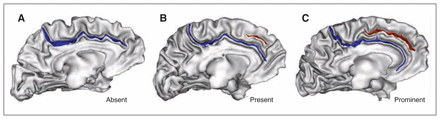
The ACC morphology was labelled using a standard 3-level classification (“absent,” “present” or “prominent”; Fig. 1) based on the paracingulate sulcus (PCS) occurrence and extent.25 The PCS was defined as the sulcus located dorsal to the cingulate sulcus with a course clearly parallel to the cingulate sulcus. We considered the PCS to be absent if there were no clearly developed horizontal sulcus elements parallel to the cingulate sulcus and extending at least 20 mm (interruptions or gaps in the PCS course were not taken into account for the length measure). The PCS was considered to be prominent if it extended greater than 40 mm and present if it extended 20–40 mm.25 We measured PCS length in Montreal Neurological Institute (MNI) space after a linear spatial normalization using a dedicated tool in BrainVISA.
Sulcal patterns of the anterior cingulate cortex (ACC). The 3 sulcal patterns of the ACC based on the occurrence and extent of the paracingulate sulcus (PCS). (A) Absent (with no PCS), (B) present (with a PCS) and (C) prominent (with a PCS > 40 mm) sulcal patterns. The cingulate sulcus (blue) and PCS (red) are represented on a 3-dimensional mesh-based reconstruction of the inner (grey matter/white matter) surface (grey) of a study patient.
We assigned an asymmetry index to each participant based on the combination of ACC sulcal pattern in the left and right hemispheres. We considered ACC morphology to be symmetrical when the ACC pattern was the same (i.e., PCS absent, PCS present or PCS prominent) in the left and right hemispheres; in all the other cases, participants were classified as asymmetrical.24
Cerebrospinal fluid volume
The CSF, white matter and grey matter volumes were automatically calculated by BrainVISA based on brain tissue segmentation; CSF volume corresponds to ventricle and sulcal CSF volume. To control for interindividual differences in total brain volume, we calculated a normalized CSF volume for each participant as the ratio between CSF volume and total brain volume. Normalized CSF volume was therefore expressed as a percentage.
Statistical analysis
We analyzed the main effects and interaction of NSS (no NSS v. NSS), handedness (right-handedness v. mixed-handedness), ACC morphology (asymmetrical v. symmetrical) and CSF volume on TMT scores using univariate linear models. Age and of years of education, as a proxy of general intelligence (IQ), were added a priori as confounding covariates in the statistical models to control for their previously detected confounding effects on TMT performance.17 Sex was also added a priori as a potential confounding factor, as some studies have reported sex-related differences in ACC asymmetry.25–27 To control for the potential bias effect of psychotic symptoms, we performed our statistical analyses using the total PANSS score as a confounding covariate.
We used Shapiro tests to check that the linear model residuals were normally distributed. Statistical significance was probed with F tests. We considered a 2-tailed p < 0.05 to be statistically significant. Part of variance explained by the model was measured using an adjusted R2. All statistical analyses were carried out using R software version 2.9 (www.r-project.org/).
Results
Of the 44 patients recruited, 41 had completed psychological assessments and MRIs and were included in our analyses. At follow-up assessment, patients met the DSM-IV diagnostic criteria for schizophrenia (n = 35), schizoaffective disorder (n = 5) or schizophreniform disorder (n = 1). Sixteen patients had never received any antipsychotic treatment before inclusion. Twenty-five patients had received a low dosage of antipsychotics (olanzapine n = 11, risperidone n = 12, clozapine n = 1, chlorpromazine n = 1); the mean lifespan cumulative chlorpromazine equivalent dose for previously treated patients was 522 ± 414 mg. The demographic and clinical characteristics of the study sample are reported in Table 1. Data on NSS, handedness, ACC morphology and CSF volume are reported in Table 2.
Demographic and clinical characteristics of the study participants (n = 41)
Neurodevelopmental markers in the patient sample (n = 41)
The mean reaction time (RT) for the TMT-A task was 34.3 ± 14.4 s. Our statistical analysis revealed a main effect of age (F = 15.9, p < 0.001) and years of education (F = 4.7, p = 0.040) as well as an interaction between handedness and CSF volume (F = 4.4, p = 0.046) on this score.
The mean RT for the TMT-B task was 80.0 ± 35.9 s. Our statistical analysis revealed an interaction between NSS and ACC morphology (F = 7.6, p = 0.011) as well as between ACC morphology and CSF volume (F = 8.0, p = 0.009) on this score.
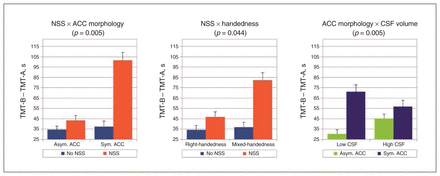
The mean RT for TMT-B – TMT-A was 45.7 ± 30.9 s. Our statistical analysis revealed a main effect of ACC morphology (F = 4.7, p = 0.041) as well as interactions between NSS and ACC morphology (F = 9.5, p = 0.005), between NSS and handedness (F = 4.5, p = 0.044) and between ACC morphology and CSF volume (F = 9.5, p = 0.005) on this differential score.
The presence of 2 markers related to neurodevelopmental deviations (i.e., presence of NSS, symmetric ACC or mixed-handedness) leads to decreased executive performances (i.e., lower TMT-B – TMT-A scores). In addition, the executive performance was lower in patients with symmetric ACC morphology than in patients with asymmetric ACC morphology, and the effect was modulated by the CSF volume: the higher the CSF volume, the less marked the difference between patients with symmetric and asymmetric ACC. Measure of the linear model adjusted R2 revealed that the combination of ACC morphology, NSS, handedness and CSF volume explained 38% of the TMT-B – TMT-A variability.
Notably, main and interaction effects remained statistically significant after controlling for PANSS total score.
In the 18 patients with follow-up assessments, there was no change between baseline and follow-up for TMT-A (30.3 ± 10.6 v. 30.3 ± 12, paired t test, p = 0.98), TMT-B (73.6 ± 32.9 v. 72.2 ± 33.1, p = 0.87), or for TMT-B – TMT-A (44.2 ± 26.0 v. 41.4 ± 29.8, p = 0.91). Notably, the baseline mean value TMT scores of this subset was similar to that of the whole sample (Table 3 and Fig. 2).
Interaction effects of markers of early brain development on the Trail Making Test (TMT) in patients with first-episode psychosis. (Left) Interaction between neurological soft signs (NSS; NSS v. no NSS) and anterior cingulate cortex (ACC) morphology (asymmetric ACC sulcal pattern v. symmetrical ACC sulcal pattern). (Middle) Interaction between NSS (NSS v. no NSS) and handedness (right-handedness v. mixed-handedness). (Right) Interaction between ACC morphology (asymmetric ACC sulcal pattern v. symmetrical ACC sulcal pattern) and sulcal/ventricle cerebrospinal fluid (CSF) volume. For visualization purposes, CSF volume was dichotomized as low and high, with the median CSF volume as the threshold. Error bars represent the standard error of the mean. The data were linearly adjusted by age, sex and years of education.
Statistical analyses of TMT scores
Discussion
This study in patients with first-episode schizophrenia reports effects of several radiological and clinical markers of early brain development (i.e., NSS, ACC sulcation, handedness and CSF volume) on CC. These markers were previously reported separately to be associated with CC deficits in patients with schizophrenia, but to our knowledge this is the first time that their cumulative as well as their interactive effects have been investigated.
In the present study we assessed CC using the TMT, a task broadly used in patients with schizophrenia, particularly in patients with first-episode psychosis.28 The TMT offers the advantage of assessing in a single test several cognitive processes: TMT-A evaluates basic processes (attention and processing speed), while TMT-B evaluates executive functions (working memory, cognitive flexibility and control inhibition). The difference score (TMT-B – TMT-A) controls for the processing speed17 and is more specific for assessing executive functioning than TMT-B alone.17
The detected main effect of ACC morphology on TMT scores is in line with the robust association between ACC morphology and CC reported both in healthy individuals24,29 and in patients with schizophrenia.14,30,31 Our analysis also reports an interaction between ACC morphology and ventricle/sulcal enlargement, indicating that the higher the enlargement, the less important the ACC morphology effect on CC. This result is consistent with the reported association between ventricle enlargement and decreased cognitive performance in patients with first-episode psychosis.13 Interactions between NSS and ACC morphology and between NSS and handedness were also detected, revealing the highest CC in patients with “typical” features (i.e., no NSS, an asymmetric ACC sulcal pattern and right-handedness) and the lowest CC in patients with “deviant” features (i.e., presence of NSS, a symmetric ACC sulcal pattern and mixed-hanedness). Our results are in line with previous findings that NSS11,32 and mixed-handedness12 are associated with executive impairments. These interactions between NSS, handedness and ACC morphology may explain the discrepancy observed in previous studies when only 1 factor was investigated without controlling for the other factors.11,33
The reported effects of different clinical and brain imaging markers related to early brain development on CC provide converging evidence of neurodevelopmental deviations associated with executive impairments in patients with schizophrenia, which is in line with previous investigations in schizophrenia.
Several developmental clinical and cognitive studies previously reported clues of deviant neurodevelopmental trajectory of executive functioning in patients with schizophrenia.10 Retrospective cognitive analyses showed that, at age 13 years, individuals in whom schizophreniform disorder subsequently developed performed significantly worse on the TMT than controls.34 In addition to an association with deviations during adolescence, TMT impairment in patients with schizophrenia has also been associated with deviations during fetal life (in utero maternal infection exposures).8 Furthermore, non–right-handedness, including mixed- and left-handedness, which is considered to result from early neurodevelopmental “failure to establish cerebral asymmetry,”23 is more frequent in patients with schizophrenia than in healthy individuals23 and is associated with executive impairments.12 Finally, NSS, referred to as the minor, nonlocalizable defects in motor coordination, motor integration and sensory integration,11 are a clinical marker of pre- and perinatal deviance;22,35 are much more frequent in patients with schizophrenia and their siblings than in controls;36 and are associated with cognitive deficits, including executive functions.11,32 The intensity of NSS may vary in the course of illness with acuity of the disorder.37 Because in our study NSS were considered a trait marker of early neurodevelopmental insult22,35 rather than a state marker of acute cerebral changes,38 we assessed NSS using categorical factors that were robust to possible changes in clinical state rather than a continuous variable.
Brain imaging studies also provided evidence of neurodevelopmental deviations in patients with schizophrenia. One of the first and most robust39 brain imaging findings in patients with schizophrenia is ventricle enlargement. Ventricle enlargement is considered to be influenced by early brain development deviations,40 is highly heritable41 and, in particular, is associated with genetic risk factors for schizophrenia.42 Initially described in patients with chronic schizophrenia, ventricle enlargement was also reported in untreated patients with first-episode psychosis.13 Notably, some studies in patients with schizophrenia also reported possible progressive changes in ventricular enlargement in the course of the disorder,39 suggesting that this anatomic feature is a marker of both early and late brain development. More recently, structural brain imaging in patients with schizophrenia focused on the sulcal pattern, a marker of fetal brain development. The sulcal patterns result from in utero processes that shape the cortex anatomy from an initially smooth lissencephalic structure to a highly convoluted surface.43 Several factors contribute to the neurodevelopmental processes that shape the folded cerebral cortex,44 including structural connectivity through axonal tension forces.44 The connectivity constraints lead to a compact layout that optimizes the transmission of neuronal signals between brain regions45 and brain network functioning. The ACC can have 2 different types of sulcal patterns, defined between 10 and 15 weeks of fetal life.46 An asymmetric pattern, with different types in the left and right hemispheres, is common in healthy individuals.25 On the contrary, there is a lack of such normal ACC asymmetry in patients with schizophrenia47–51 and in individuals at high risk for schizophrenia.31,52–54 This observation is line with the hypothesis that anomalies of brain asymmetry are a key feature of schizophrenia.55
The results of this study are best understood in the context of some methodological issues. Age and years of education, as a proxy for general intelligence (IQ), were added a priori in the analyses as confounding factors because of their previously reported effects on TMT scores.17 The negative association between age and years of education on TMT-A scores is in accordance with the decreased attention and processing speed17 observed with age and lower general intelligence in patients with first-episode psychosis.28,56 Our study focused on patients with first-episode schizophrenia to exclude effects due to chronic evolution of the disease. In the present study, patients were untreated or treated for only a few days, thus limiting the possible effects of medication.
Longitudinal assessment was available in a subsample of the patients and showed an overall stability of cognitive control assessed by TMT scores. This longitudinal stability is in agreement with previous studies showing that executive impairments are stable over time. For instance, Sanchez-Torres and colleagues57 found that TMT scores were stable for 10 years in 85% of patients. Additionally, adding the PANSS total score as a cofounding covariate in our statistical analyses did not change our findings, suggesting that the influence of symptoms (“state”), if any, on cognitive control is minimal. Taken together, these findings support the notion that cognitive control, as measured with the TMT, can be considered a trait marker of schizophrenia.
Limitations
No 3-way nor 4-way interactions were detected among the 4 neurodevelopmental factors. The sample size (n = 41) was clearly adapted to detect main effects but may have limited the statistical power to investigate 3-way or 4-way interactions. Our results may be further completed in a larger sample, which would allow the detection of more subtle interactive effects.
Our analyses were based on a global measure of CSF volume. The differential contribution of cortical as well as subcortical atrophy to CSF versus ventricular volume could therefore not be investigated.
This study focused on the morphology of ACC as this region plays a key role in cognitive control. The morphology of other brain regions may also have an impact on cognitive control.58 We could not, however, perform whole-brain analysis with the approach used in this study because only a few cortical regions, including the ACC, present qualitatively distinct sulcal patterns. In addition, our approach requires visual inspection, which is very time-consuming and cannot be easily performed in multiple areas.
To avoid the known effects of medications and chronic evolution on cognition, our study included drug-naive patients with first-episode psychosis. Several previous studies similarly assessed cognitive functioning in drug-naive patients with psychosis (for example, see the studies by Chan and colleagues59 and Rodríguez-Sánchez and colleagues60), and their results support that patients with first-episode psychosis can undergo brief cognitive assessment. Nonetheless, we enrolled only patients who were able to give their consent to receive further treatment. This may have led to a potential bias by excluding patients with more severe symptoms.
Conclusion
Our findings support the neurodevelopmental model of schizophrenia but also provide new insight regarding deviations during the early stages of brain development. Our findings suggest that executive deficits in patients with schizophrenia are not associated with a single impaired neurodevelopmental process during fetal life but rather correspond to the common final pathway of several impaired processes. The detected cumulative and interactive effects of the 4 clinical and radiological markers suggest that each marker captures different aspects of early brain deviation. The classical model that emerges from the neurodevelopmental perspective is that of an “early insult,” a latent period through much of neural development, and the emergence of psychosis in late adolescence or early adulthood.2 Our findings extend this view by suggesting that it is not a single early insult, but rather several early insults that confer vulnerability for schizophrenia. Our study raises the speculation that several early brain development deviations, reflected by different clinical and radiological markers, may interact, likely nonlinearly, and subsequently lead to executive impairments.
Acknowledgements
The authors thank the CERC and SHU teams for their help during patient enrolment and data management. This work was supported by INSERM, ANR/ERANET-NEURON (AUSZ_EUCan), Académie de Médecine (O. Gay), Fondation Deniker, Fondation NRJ and Fondation Houiez (A. Cachia).
Footnotes
↵* These authors contributed equally to this work.
Competing interests: None declared.
Contributors: O. Gay, M. Plaze, M.-O. Krebs and A. Cachia designed the study. O. Gay, M. Plaze, C. Oppenheim, R. Gaillard, J.-P. Olié and M.-O. Krebs acquired the data, which O. Gay, M. Plaze, M.-O. Krebs and A. Cachia analyzed. O. Gay, M. Plaze, M.-O. Krebs and A. Cachia wrote the article, which all authors reviewed and approved for publication.
- Received August 4, 2015.
- Revision received February 10, 2016.
- Revision received May 2, 2016.
- Accepted May 16, 2016.