Abstract
Background: Cognitive behavioural therapy (CBT), including exposure and ritual prevention, is a first-line treatment for obsessive–compulsive disorder (OCD), but few reliable predictors of CBT outcome have been identified. Based on research in animal models, we hypothesized that individual differences in basolateral amygdala–ventromedial prefrontal cortex (BLA–vmPFC) communication would predict CBT outcome in patients with OCD.
Methods: We investigated whether BLA–vmPFC resting-state functional connectivity (rs-fc) predicts CBT outcome in patients with OCD. We assessed BLA–vmPFC rs-fc in patients with OCD on a stable dose of a selective serotonin reuptake inhibitor who then received CBT and in healthy control participants.
Results: We included 73 patients with OCD and 84 healthy controls in our study. Decreased BLA–vmPFC rs-fc predicted a better CBT outcome in patients with OCD and was also detected in those with OCD compared with healthy participants. Additional analyses revealed that decreased BLA–vmPFC rs-fc uniquely characterized the patients with OCD who responded to CBT.
Limitations: We used a sample of convenience, and all patients were receiving pharmacological treatment for OCD.
Conclusion: In this large sample of patients with OCD, BLA–vmPFC functional connectivity predicted CBT outcome. These results suggest that future research should investigate the potential of BLA–vmPFC pathways to inform treatment selection for CBT across patients with OCD and anxiety disorders.
Introduction
Predicting who will or will not respond to a particular treatment is a priority for the development of more personalized interventions in mental health. Functional neuroimaging measures may help achieve this goal.1 For example, greater activity at baseline in the anterior insula or anterior cingulate cortex (ACC), measured with 18F-fluorodeoxyglucose positron emission tomography (FDG-PET), has been associated with a positive outcome from antidepressant medication in patients with major depressive disorder.2,3 In the present study we tested whether resting-state functional connectivity (rs-fc) between the basolateral amygdala (BLA) and ventromedial prefrontal cortex (vmPFC) could predict outcome from cognitive behavioural therapy (CBT) in patients with obsessive–compulsive disorder (OCD).
Cognitive behavioural therapy, including exposure and ritual prevention, is a first-line treatment for OCD, but some patients do not respond or respond only partially to CBT.4 There have been several attempts to correlate functional neuroimaging measures and CBT outcomes in patients with OCD.1,5 Initial studies focused on cortico–striato–thalamo–cortical regions believed to be involved in the pathophysiology of OCD, such as the orbitofrontal cortex (OFC). Indeed, findings from studies using FDG-PET or single-photon emission computed tomography (SPECT) suggest that greater baseline OFC activity in patients with OCD is associated with a positive outcome from CBT. However, findings from functional MRI (fMRI) studies do not consistently show that pretreatment frontal or striatal activation is associated with CBT outcome.1
Newer conceptualizations emphasize the contribution of the amygdala and vmPFC in OCD pathophysiology and treatment.6 Both regions are involved in fear processing and regulation, and both play a role in extinction learning, a likely mechanism of action in CBT for fear-related disorders, including OCD.7 The amygdala consists of several functionally distinct nuclei, with 2 broad subdivisions: the BLA and the centromedial amygdala (CMA). Although both are involved in fear processing, animal studies suggest that the BLA has a more prominent role in fear learning (including fear conditioning and extinction) and that the CMA has a more prominent role in fear expression.8 In healthy humans, rs-fc of these 2 regions differ: spontaneous BLA activity correlates with activity in temporal and frontal cortical regions (including the vmPFC), whereas spontaneous CMA activity correlates with activity in the striatum.9 The vmPFC is associated with different emotional and cognitive processes, and this complex role is likely supported by its connectivity with various regions (e.g., striatum, amygdala).10 Data from animals and humans suggest that vmPFC connections with the amygdala form a network important for regulating stress and aversive responses. More specifically, the vmPFC seems to exert inhibitory control over BLA activation, thereby modulating its response to aversive stimuli.11
Dynamic communication between the BLA and vmPFC has been proposed as a key cross-species mechanism in adaptive learning, with deficient communication across regions resulting in maladaptive learning and fear generalization.12 Thus, individual differences in the functioning of the BLA–vmPFC circuit may predict the outcome of learning-based treatments such as CBT. However, little research has focused on connectivity within this specific circuit. Using rs-fc and an exploratory whole-brain approach, a recent study in patients with OCD found that the degree centrality (a generally accepted marker of functional connectivity) in the right BLA was positively associated with CBT outcome.13 That study, however, did not assess connectivity at the network level (i.e., it did not provide information about connectivity between the BLA and frontal regions). Moreover, it used a small sample (n = 17) and studied only inpatients with OCD, thus limiting the generalizability of the findings. Another study in patients with social anxiety disorder (SAD) found that rs-fc between the right amygdala and the pregenual anterior cingulate cortex (pgACC) and between the left amygdala and the pgACC/medial prefrontal cortex was positively associated with CBT outcome.14 That study also used a small sample (n = 21) and did not assess connectivity from amygdalar subregions. Moreover, no control group was included, precluding the assessment of baseline abnormalities in rs-fc.
Capitalizing on a large sample of patients with OCD who were receiving a stable dose of a selective serotonin reuptake inhibitor (SSRI) and who then received CBT, we tested the hypothesis that BLA–vmPFC rs-fc would predict CBT outcome. In exploratory analyses, we tested whether these findings would be specific to BLA–vmPFC (rather than other amygdala–prefrontal cortex circuits) and whether BLA–vmPFC rs-fc would differ between patients with OCD and healthy control participants.
Methods
Participants
We recruited patients from the obsessive–compulsive disorders unit of the University Hospital of Bellvitge, Barcelona, Spain. We recruited healthy controls (group-matched by age, sex and years of education) in the local community through advertisements and word of mouth. All participants provided written informed consent after receiving a complete description of our study protocol, which was approved by the Institutional Review Board of the University Hospital of Bellvitge.
Eligible patients were adults with a principal diagnosis of OCD (≥ 1 year) who were enrolled in our unit for an open 12-week pharmacological trial with an SSRI (clomipramine, fluoxetine, fluvoxamine, or escitalopram). Patients were eligible for CBT and this fMRI study if they did not respond (Yale–Brown Obsessive Compulsive Scale [YBOCS] reduction < 25%) or showed only partial response (YBOCS reduction < 35%)15 after at least 12 weeks of a stable dose of the SSRI, following recommended guidelines.16 None had previously received CBT. The time between the end of the pharmacological trial and the initiation of CBT ranged between 1 and 2 weeks; the fMRI data were collected during this 1–2 week interval. Concomitant psychotropic medications were not permitted (except benzodiazepines if used only for sleep).
Exclusion criteria for all participants were substance use, abuse or dependence; psychotic or bipolar disorders; mental retardation; presence or history of serious medical or neurologic disorder (except tic disorder); or any contraindication to MRI scanning. Patients with OCD who had comorbid non-psychotic mood and anxiety disorders were included, provided that OCD was the principal and most severe diagnosis.
Psychiatric diagnoses in the OCD group were established independently by 2 psychiatrists (P.A. and C.S.) using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-IV), Clinician Version.17 To be included in the healthy control group, participants had to have no lifetime Axis I disorders, as established by a psychiatrist (E.R. or M.S.) using the SCID-IV, Non-patient Edition.18
Data from the same OCD and control samples were included in a previous study of structural MRI predictors of CBT outcome, including cortical thickness.19
Neuroimaging data acquisition, preprocessing and analyses
Image acquisition
Participants were scanned on a 1.5 T scanner (Signa Excite system; General Electric) equipped with an 8-channel phased-array head coil. The functional sequence consisted of gradient recalled acquisition with the following parameters: repetition time (TR) 2000 ms, echo time (TE) 50 ms, flip angle 90°, field of view (FOV) 24 cm, with a 64 × 64 pixel matrix and a slice thickness of 4 mm. Twenty-two interleaved sections, parallel to the anterior–posterior commissure line, were acquired to generate 120 whole brain volumes, excluding 4 initial dummy volumes (total scan time 4 minutes). Participants were instructed to relax, stay awake and to lie still while keeping their eyes closed throughout the scan. A high-resolution T1-weighted anatomic image was also obtained from each participant using a 3-dimensional fast spoiled gradient inversion-recovery prepared sequence with 130 contiguous slices in the axial plane with the following parameters: TR 11.8 ms, TE 4.2 ms, flip angle 15°, FOV 30 cm, with a 256 × 256 pixel matrix and a slice thickness of 1.2 mm.
Image preprocessing
Standard image preprocessing was conducted using SPM12 (http://www.fil.ion.ucl.ac.uk/spm) and the CONN-fMRI Functional Connectivity toolbox version 1320 with MATLAB version R2012b. All functional images were slice time– corrected, motion-corrected using a 6-parameter rigid body transformation, and then coregistered to each participant’s T1-weighted structural image. Coregistered images were normalized to the Montreal Neurological Institute (MNI) canonical template and smoothed with an 8 mm full-width at half-maximum Gaussian kernel. Preprocessing procedures also included band-pass filtering with a frequency window of 0.01–0.09 Hz and outlier detection using Artifact Detection Tools implemented in CONN. The principal component-based noise-correction “CompCor” method was implemented with this toolbox21 to additionally control for physiologic noise and head motion artifacts. Within each participant, volumes having large spiking (i.e., > 3 standard deviations from the mean image intensity) or large motion artifacts (i.e., 0.5 mm for scan-to-scan head motion composite changes in the x, y, or z direction) were classified as outliers.
Anatomic images were segmented into grey matter, white matter and cerebrospinal fluid (CSF) regions. Head motion (6 realignment parameters and their derivatives), outlier classification, and the blood oxygen level–dependent (BOLD) time series from the participant-specific white matter and CSF masks were used as nuisance regressors and removed from the BOLD functional time series using linear regression at the individual participant level. No participants had head movement exceeding ± 3 mm or more than 20% of the data points classified as outliers.
Functional connectivity analyses
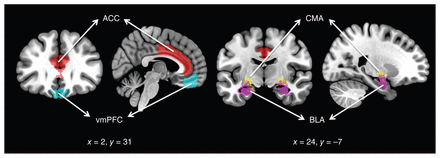
Following preprocessing, we performed rs-fc analyses using the following regions of interest (ROIs) bilaterally: the BLA, CMA, vmPFC and ACC. Amygdala subregions of interest (BLA and CMA) were derived from the Juelich Histological Atlas22 with 6935 voxels in each side of the BLA and 2878 voxels in each side of the CMA after normalization to a standard template (2 × 2 × 2). Cortical ROIs (the vmPFC and ACC) were derived from the FSL Harvard–Oxford atlas maximum likelihood cortical atlas with a 70% threshold.23 Figure 1 shows the ROIs used in the present study. As seen in the figure, the vmPFC included the medial orbitofrontal cortex, but excluded the subgenual region. The mean BOLD time series was computed across all voxels within each ROI, and Pearson correlation coefficients were calculated to determine the linear association of the BOLD time series between each pair of regions for each participant. The resulting correlation coefficients were transformed into z-scores using Fisher transformation to satisfy normality assumptions. These rs-fc values for each participant were then used in our group analyses.
Regions of interest used in the study. ACC = anterior cingulate cortex; BLA = basolateral amygdala; CMA = centromedial amygdala; vmPFC = ventromedial prefrontal cortex.
Cognitive behavioural therapy and assessment of outcome
Cognitive behavioural therapy focused on exposure and ritual prevention, was manualized24 and provided by an experienced therapist who was blind to the study’s hypotheses. All patients received 20 individual weekly sessions lasting approximately 45 minutes each. The first 2 sessions were devoted to psychoeducation, the introduction to the behavioural model of OCD and the development of an exposure hierarchy. Sessions 3–18 consisted of gradual exposure to items of the hierarchy, during which patients faced their obsessional fears for a prolonged period without ritualizing. The goal was for patients to stop their rituals as early as possible during treatment. Formal cognitive therapy techniques were not used, but dysfunctional cognitions were discussed within the context of exposure. Between sessions, homework (60 minutes daily) consisting of exposure to stimuli similar to those addressed in the sessions was assigned. The final 2 sessions were devoted to relapse prevention. The SSRI medication was kept at a stable dose throughout the study.
Clinicians not involved in treatment assessed OCD symptoms and comorbid depressive symptoms before and after CBT using the YBOCS25 and the Hamilton Rating Scale for Depression (HAMD).26 We operationalized CBT outcome both as a categorical (i.e., response v. nonresponse to CBT based on ≥ 35% decrease in the YBOCS total score)15 and a continuous (post-CBT YBOCS total score) variable.
Statistical analyses
To test our a priori hypothesis that BLA–vmPFC rs-fc would predict CBT outcome in patients with OCD, our primary analyses consisted of a binomial logistic regression analysis with CBT response (yes v. no) as the dependent variable as well as a multiple regression analysis with post-CBT YBOCS as the dependent variable. In both the regression and the logistic regression analyses, BLA–vmPFC rs-fc was the predictor, and we adjusted for age, sex, OCD symptoms (baseline YBOCS) and depressive symptoms (baseline HAMD) as, based on previous research,27 these covariates may be associated with CBT outcome. Adjusting for these variables also reduces noise in the regression models, thereby increasing statistical power. In exploratory analyses, we tested the specificity of these results by repeating the same analyses using CMA–vmPFC and BLA–ACC rs-fc. Finally, we explored differences in BLA–vmPFC rs-fc across patients with OCD and healthy controls using 1-way analysis of covariance (ANCOVA), adjusting for age and sex. We report findings from the 2 primary analyses that were significant after Bonferroni correction and exploratory findings that were significant after Benjamini–Hochberg correction for preserving the false-discovery rate.
Results
Participants
Of the initial sample of 83 eligible patients with OCD, 3 refused to initiate CBT and 6 dropped out before completing the first 5 sessions. In addition, 1 patient and 2 of the 86 recruited healthy controls were excluded for technical reasons (corrupted data), leaving 73 patients and 84 controls for analysis. The demographic and clinical characteristics of the sample are shown in Table 1.
Sociodemographic and clinical characteristics of study participants
Response to CBT
After their completion of CBT, we considered 35 (48%) patients with OCD to be responders and 38 (52%) to be nonresponders. Responders and nonresponders did not differ significantly on any sociodemographic or clinical variables (including the frequencies of type and dose of SSRI treatment; Appendix 1, Table S1, available at jpn.ca/160215-a1) other than baseline YBOCS score, which was lower in CBT responders than in non-responders (mean 20.43 ± 4.88 v. 23.68 ± 4.81, t71 = 2.86, p = 0.005).
Does BLA–vmPFC rs-fc predict CBT outcome in patients with OCD?
Our binomial logistic regression model with CBT response (yes v. no) as the dependent variable was statistically significant (χ2 = 16.246, p = 0.003). Decreased BLA–vmPFC rs-fc significantly predicted a better CBT outcome (odds ratio [OR] 0.032, Wald test = 5.246, p = 0.022, Bonferroni-adjusted p = 0.044). Our multiple regression model with YBOCS score following CBT as the dependent variable was also statistically significant (F5,67 = 28.193, p < 0.001, adjusted R2 = 0.654). Again, decreased BLA–vmPFC rs-fc predicted a better CBT outcome (B = 7.42, t = 3.095, p = 0.003, Bonferroni-adjusted p = 0.006, Table 2). The same findings emerged when we used the difference between pre- and post-CBT YBOCS scores as the dependent variable, either controlling or not controlling for pre-CBT YBOCS (p = 0.003). Of note, BLA–vmPFC rs-fc was not associated with baseline OCD severity (pre-CBT YBOCS score: r = 0.068, p = 0.57) or illness duration (r = –0.016, p = 0.89).
Regression models predicting CBT outcome in patients with OCD (n = 73) with basolateral amygdala–ventromedial prefrontal cortex resting-state functional connectivity as a predictor
Are these findings specific to BLA–vmPFC rs-fc?
Neither CMA–vmPFC rs-fc nor BLA–ACC rs-fc significantly predicted CBT outcome using our categorical (yes v. no) outcome measure (CMA–vmPFC rs-fc: OR 0.065, Wald test = 1.956, p = 0.16, Benjamini–Hochberg-adjusted p = 0.26; BLA–ACC rs-fc: OR 1.777, Wald test = 0.151, p = 0.70, Benjamini–Hochberg-adjusted p = 0.80). Using our continuous (post-CBT YBOCS score) measure as outcome, CMA–vmPFC rs-fc was a significant predictor (B = 8.489, t = 2.518, p = 0.014, Benjamini–Hochberg-adjusted p = 0.028), but BLA–ACC rs-fc was not (B = −0.362, t = −0.131, p = 0.90, Benjamini–Hochberg-adjusted p = 0.90; Appendix 1, Tables S2 and S3).
Does BLA–vmPFC rs-fc differ between patients with OCD and healthy controls?
The results of our ANCOVA showed that BLA–vmPFC rs-fc differed significantly between patients with OCD and healthy controls (F1,153 = 9.676, p = 0.002, Benjamini–Hochberg-adjusted p = 0.005, partial η2 = 0.05), with decreased BLA–vmPFC rs-fc in the OCD group (mean difference = −0.103, 95% confidence interval [CI] −0.169 to −0.038, p = 0.002, Benjamini–Hochberg-adjusted p = 0.005).
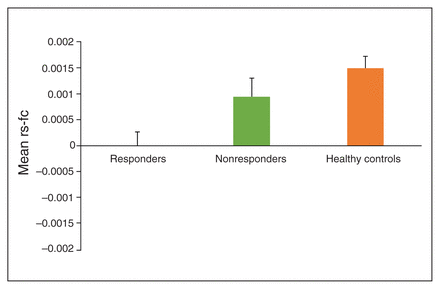
To examine whether decreased BLA–vmPFC rs-fc characterized OCD per se or whether it decreased CBT response capacity, we conducted an additional ANCOVA comparing BLA–vmPFC rs-fc in CBT responders, CBT nonresponders and healthy controls, also adjusting for age and sex. This analysis revealed a main effect of group (F2,152 = 6.895, p = 0.001, Benjamini–Hochberg-adjusted p = 0.005, partial η2 = 0.08. Post hoc comparisons revealed significantly decreased BLA–vmPFC rs-fc in CBT responders compared with healthy controls (mean difference −0.153, 95% CI −0.235 to −0.071, Fisher least significance difference [LSD] p < 0.001) and CBT nonresponders (mean difference −0.096, 95% CI −0.191 to −0.0002, Fisher LSD p = 0.049). Additionally, BLA–vmPFC rs-fc was similar across CBT nonresponders and healthy controls (mean difference −0.057, 95% CI −0.137 to −0.022, Fisher LSD p = 0.16). Thus, decreased BLA–vmPFC rs-fc was uniquely characteristic of participants with OCD who responded to CBT (Fig. 2), consistent with our finding that BLA–vmPFC rs-fc was not significantly associated with baseline OCD severity.
Mean values of resting-state functional connectivity (rs-fc) between the basolateral amygdala and ventromedial prefrontal cortex (BLA–vmPFC) in patients with obsessive–compulsive disorder (OCD) who responded to cognitive behavioural therapy (CBT; n = 35), patients with OCD who did not respond to CBT (n = 38) and healthy controls (n = 84). Responders to CBT showed significantly decreased BLA–vmPFC rs-fc in comparison to healthy controls (p < 0.001) and CBT nonresponders (p = 0.05). Bars represent standard error of the mean.
Discussion
We investigated rs-fc between the amygdala and prefrontal cortex subregions in a large sample of patients with OCD and healthy participants. Our hypothesis was confirmed: BLA–vmPFC connectivity was significantly associated with CBT outcome, with decreased connectivity predicting a better outcome. These findings were relatively specific to BLA–vmPFC connectivity in that they were not replicated with measures of BLA–ACC (for either outcome measures) and were only replicated with CMA–vmPFC connectivity when our continuous outcome measure was used. Decreased BLA–vmPFC connectivity also characterized patients with OCD in comparison to healthy participants, but was specific to the patients with OCD who responded to CBT.
Our finding that BLA–vmPFC functional connectivity is associated with CBT outcome is consistent with previous findings showing associations of BLA degree centrality with CBT outcome in a much smaller sample of patients with OCD13 and of amygdala–prefrontal functional connectivity with CBT outcome in patients with SAD (although in the opposite direction).14 Our results add specificity to these previous findings, showing that BLA–vmPFC connectivity best predicts CBT outcome, rather than “overall” connectivity of the BLA, as measured by the degree centrality, or connectivity of the entire amygdala.
Although our comparison between patients and controls suggests that BLA–vmPFC functional connectivity may be abnormal in patients with OCD, as previously shown in other fear-related disorders,28 our comparison among CBT responders, nonresponders and controls indicated that baseline BLA–vmPFC connectivity may differ within patients with OCD who do or do not respond to CBT. Moreover, in our study, BLA–vmPFC connectivity was not associated with baseline OCD severity, suggesting that BLA–vmPFC connectivity is a biomarker of responsivity to CBT rather than a marker of OCD psychopathology. Consistent with findings from the above-mentioned SAD study, these data suggest that measures of amygdala–prefrontal connectivity may predict CBT outcome across different fear-related disorders. Of note, amygdala–prefrontal connectivity was positively associated with CBT outcome in patients with SAD,14 but inversely associated with outcome in patients with OCD.
Our findings suggest that CBT may have a restoring effect on the cortical inhibition of limbic activity. This interpretation is consistent with findings from task-based fMRI studies of depression in which participants with the least pretreatment subgenual ACC activation showed a better outcome with CBT,29,30 suggesting that CBT may be more helpful to those patients who cannot (at baseline) effectively engage brain areas supporting emotional regulation. Similarly, our findings suggest that patients with OCD with less cortical inhibition (of the vmPFC over the BLA) at baseline might benefit most from the putative restoring effects of CBT.
Our interpretation of BLA–vmPFC functional connectivity as a marker of responsivity to CBT is consistent with the well-established role of this circuit in adaptive learning.12 What is unclear, however, is how this circuit mediates CBT outcome. One possible explanation is that BLA–vmPFC connectivity represents an indirect measure of individual differences in fear extinction capacities. This explanation is consistent with recent findings that brain metabolism in the amygdala (as measured with FDG-PET) predicts fMRI activation of the vmPFC during fear extinction.31 Moreover, the better predictive value of BLA–vmPFC over CMA–vmPFC in our study is consistent with animal and human data suggesting the pre-eminent role of the BLA in fear extinction learning.12,32 The association of CMA–vmPFC connectivity with our continuous measure of CBT outcome could reflect the association of CMA with fear extinction expression.33 However, amygdala–prefrontal circuits are involved in other processes, such as attentional regulation and the interpretation of emotional stimuli,34 and these processes may also be associated with CBT response.
Our previous finding that less cortical thickness in the rostral ACC was inversely associated with CBT outcome in this same sample19 suggests that cortical regions from the ventral–anterior medial wall play an important role in predicting CBT, which can be apparent using different neuro-imaging measures. It is possible that these measures index different aspects (e.g., attentional v. cognitive) of the same process (fear extinction).
The main implication of our findings is that examining the BLA–vmPFC circuit may offer important insights in the prediction of CBT outcome for patients with OCD and, potentially, patients with other fear-related disorders. Moreover, as baseline OCD severity was unassociated with BLA–vmPFC connectivity and was controlled for in our analyses, our findings further suggest that such corticolimbic connectivity may account for variability in CBT outcome beyond OCD baseline severity. Functional connectivity is relatively easy to acquire, has relatively high test–retest reliability35 and does not rely on a patient’s ability to perform a task, which may favour its translation to the clinic. Strengths of this study include our clear hypothesis-driven approach based on previous translational research, the large sample of patients and controls and our analysis of specificity.
Limitations
A few limitations should be noted. First, we used a sample of convenience and did not include a control group of patients who were not receiving treatment. Thus, what is unknown is whether our findings are specific to CBT or whether they also extend to other psychological treatments. Second, we did not assess posttreatment changes in rs-fc, thereby precluding our understanding of functional connectivity changes associated with treatment. Third, all patients were receiving pharmacological treatment (SSRIs) for OCD. Patients who remained symptomatic following drug treatment were included in this study because most patients with OCD are unlikely to receive CBT as the first treatment option,36 making our administration of CBT in this study akin to real clinical practice and consistent with the use of CBT as an augmentation to drug treatment in nonresponders or partial responders.16 Although the potential influence of medication on our results cannot be ruled out, medication effects would be similar across responders and nonresponders as both groups had almost identical pharmacological treatments. Other limitations include our use of a 1.5 T scanner and a relatively short resting-state scanning time. Although a 1.5 T scanner is adequate for fMRI37 and for visualizing amygdala subregions (e.g., see the study by Vogel and colleagues38), this study warrants replication at higher resolution and with longer scan durations. Moreover, although the study therapists were highly experienced and followed a treatment manual, we did not evaluate fidelity to the manual or assess treatment compliance among patients. Finally, we did not assess specific OCD symptom dimensions, which have been shown to modulate brain findings and treatment outcome in some studies.39
Conclusion
Our data suggest that a translationally guided and relatively easy to collect neuroimaging assessment can be a significant, relatively specific and clinically useful predictor of CBT outcome in patients with OCD. Future studies should test the predictive capacity of the same measure used here in unmedicated patients and test the specificity of the findings in patients with other disorders in which CBT is used and who are receiving other treatments (e.g., medication or non-CBT-based psychological treatments). If confirmed, the findings from our study could ultimately contribute to more personalized therapies for patients with OCD. On a broader level, they could help match treatments that rely on basolateral amygdala–ventromedial prefrontal pathways to those patients who will be most likely to benefit from them.
Footnotes
↵* Share first authorship;
↵† share senior authorship.
Funding: Funding for this study was provided by Instituto de Salud Carlos III (ISCIII) (PI12/01306, PI13/01958, PI14/00413, PI16/00889), FEDER funds/European Regional Development Fund (ERDF) — a way to build Europe — and AGAUR (2014 SGR 1672). CIBERSAM is an initiative of the Carlos III Health Institute. E. Real was supported by a Juan Rodés contract (JR14/00038), M. Subirà by a Rio Hortega contract (CM15/00189) and C. Soriano-Mas by a Miguel Servet contract (CPII16/00048) from the ISCIII. C. López-Solà was supported by the Ministerio de Educación, Cultura y Deporte de España (FPU12/01636). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Competing interests: H. Simpson reports royalties from UpToDate, Inc. and Cambridge University Press outside the submitted work.
Contributors: M. Fullana designed the study. P. Alonso, N. Cardoner, E. Real, C. López-Solà, C. Segalàs, M. Subirà and J. Menchón acquired the data, which M. Fullana, X. Zhu, H. Gafalvy, J. Menchón, H. Simpson, R. Marsh and C. Soriano-Mas analyzed. M. Fullana, H. Simpson, R. Marsh and C. Soriano-Mas wrote the article, which all authors reviewed and approved for publication.
- Received November 7, 2016.
- Revision received March 24, 2017.
- Accepted April 18, 2017.