Abstract
Background: Deficits in cortical inhibitory processes have been suggested as underlying pathophysiological mechanisms of obsessive–compulsive disorder (OCD). We examined whether patients with OCD have altered cortical excitability using paired-pulse transcranial magnetic stimulation (TMS). We also tested associations between TMS indices and OCD-related characteristics, including age of onset and response inhibition in the go/no-go paradigm, to examine whether altered cortical excitability contributes to symptom formation and behavioural inhibition deficit in patients with OCD.
Methods: We assessed motor cortex excitability using paired-pulse TMS in 51 patients with OCD and 39 age-matched healthy controls. We also assessed clinical symptoms and response inhibition in the go/no-go task. All patients were undergoing treatment with serotonin reuptake inhibitors. We performed repeated-measures multivariate analysis of covariance to compare TMS indices between patients with OCD and controls.
Results: Compared to controls, patients with OCD showed a shorter cortical silent period and decreased intracortical facilitation. However, we found no significant difference between groups for resting motor threshold or short-interval intracortical inhibition. In the OCD group, the shortened cortical silent period was associated with a prompt reaction time in the go/no-go task and with early onset of OCD.
Limitations: We could not exclude the influence of medications on motor cortex excitability.
Conclusion: These findings suggest abnormal cortical excitability in patients with OCD. The associations between cortical silent period and response inhibition and age of onset further indicate that altered cortical excitability may play an important role in the development of OCD.
Introduction
Obsessive–compulsive disorder (OCD) is a neuropsychiatric disorder characterized by distressing, time-consuming or impairing obsessions (recurrent thoughts, images or urges) and compulsions (repetitive behaviours or mental acts).1 Several lines of evidence support the concept that dysfunctions of inhibitory control in frontostriatal circuits are associated with the inability to inhibit cognition and behaviour, as well as increased action-monitoring in people with OCD.2–4 Behavioural studies have shown that people with OCD have impaired motor and cognitive inhibitory mechanisms.5–7 People with OCD have shown impaired response inhibition in behavioural inhibition tasks such as the go/no-go task,5 and young people with OCD have revealed deficits of behavioural inhibition in an oculomotor task.6 In addition, neuroimaging studies have shown that excessive activation in specific brain regions, including the anterior cingulate and orbitofrontal cortices, in patients with OCD during trials required response inhibition.8,9 Accumulating evidence has suggested that altered cortical inhibition, such as an imbalance of direct and indirect feedback loops within cortical–striatal–thalamic–cortical (CSTC) circuits, may contribute to the characteristic cognitive disruptions of OCD.2,10 An optogenetic mouse model has shown that stimulation in the frontostriatal pathway can alleviate OCD-relevant behaviours. 11 These findings indicate that deficits in cortical inhibition in motor and cognitive processes may play a key role in the mechanisms of behavioural inhibition deficit and symptom formation in patients with OCD.
Paired-pulse transcranial magnetic stimulation (TMS) is a noninvasive technique that allows researchers to directly assess cortical excitability, which depends on the balance between excitatory and inhibitory circuits. Paired-pulse TMS with subthreshold conditioning can test cortical excitability directly by measuring short-interval intracortical inhibition (SICI) at interstimulus intervals (ISIs) of 1 ms to 4 ms and intracortical facilitation (ICF) at longer ISIs of 6 ms to 20 ms.12,13 A growing body of evidence suggests that SICI is mediated by γ-aminobutyric acid (GABA)-A receptors,14 while ICF depends, in part, on glutamatergic neurotransmission.15,16 Another key measure of TMS is the cortical silent period (CSP), which reflects GABA-mediated motor cortical postsynaptic inhibition. It has been suggested that short CSPs elicited by low stimulus intensity are associated with the activation of GABA-A receptors, while long CSPs elicited by high stimulus intensity are associated with the activation of GABA-B receptors.17 These TMS parameters can be a promising neurophysiological biomarker for elucidating the underlying mechanisms of psychiatric disorders that involve GABAergic and glutamatergic neurotransmission.
To date, inhibitory deficits and enhanced intracortical facilitation have been implicated in OCD, with a limited number of studies examining cortical excitability using paired-pulse TMS.18 One study reported that in a small OCD sample, patients showed significantly reduced SICI and a decreased motor threshold compared with healthy controls.19 In contrast, a subsequent study in a healthy population reported that decreased SICI may be more linked to anxiety and depression personality traits than to OCD itself.20 Another study in patients with OCD (n = 34) showed that OCD was associated with shortened CSP and increased ICF, and not associated with SICI, suggesting that dysregulation of GABA-B and N-methyl-d-aspartate (NMDA) receptor-mediated neurotransmission may be involved in the pathophysiology of OCD.21
We aimed to investigate differences in cortical excitability between patients with OCD and healthy controls using paired-pulse TMS. We also examined associations between TMS indices and clinical characteristics — including age of onset and response inhibition in the go/no-go paradigm — to examine whether altered cortical excitability contributes to symptom formation and behavioural inhibition deficit in patients with OCD. We hypothesized that patients with OCD would have abnormal cortical excitability with reduced resting motor threshold (RMT), shortened CSP, reduced SICI or increased ICF and would show correlations between neurophysiological alteration and specific OCD-related characteristics.
Methods
Participants
We recruited 55 patients with OCD from the OCD clinic in Severance Hospital and Yonsei Phil Neuropsychiatric Clinic, and 42 age-matched healthy controls via advertising. Participants underwent a face-to-face interview based on the Structured Clinical Interview for DSM-IV disorders.22 Inclusion criteria for the patient group were age 18 to 50 years, a current DSM-IV diagnosis of OCD and stable maintenance of medication for at least 8 weeks before enrollment. Patients with comorbid depression but without psychotic features were included in the study only if the obsessive–compulsive symptoms were their most prominent symptoms and the onset of OCD predated the onset of depression. Controls were included if they had been physically healthy and medication-free for the past 6 months, had no history of psychiatric disorders (including OCD and depressive disorders) and no family history of psychiatric disorders among first-degree relatives. All participants were right-handed. Participants were excluded if they presented with a movement disorder other than a tic; any psychotic symptoms; other anxiety disorders; an intellectual disability; alcohol or other substance abuse within the last 6 months; or a history of seizure, psychosurgery, encephalitis or significant head trauma. The study was approved by the ethical committee of Severance Hospital, and written informed consent was obtained from all participants.
Among the recruited participants, 2 patients and 2 controls with comorbid psychiatric problems were excluded. Two patients and 1 control who felt uncomfortable during the TMS procedure and wanted to stop participation (2 headache, 1 nonspecific discomfort) were also excluded because of incomplete acquisition of TMS data. Ultimately, 51 patients with OCD (mean age ± standard deviation = 27.43 ± 7.64 years) and 39 age-matched healthy controls (27.36 ± 6.99 years) were included in our analyses. The present sample exceeded the recommended sample size of 34 in each group required to detect the global effect with medium effect size in a multivariate analysis of variance according to G*Power.23
TMS protocol and stimulation parameters
For TMS, we used 2 Magstim-200 stimulators connected via a Bistim module with a 70 mm figure-8 coil (Magstim Company Ltd.). We took TMS recordings with surface electrodes from the abductor digiti minimi muscle. The coil was held with the grip pointing backward and perpendicular to the central sulcus. For optimal coil positioning, we measured the amplitudes of the motor evoked potential in the resting abductor digiti minimi by moving the coil in 1 cm steps over the presumed area of the contralateral motor cortex. We performed the paired-pulse paradigms on both hemispheres.
For measures of motor cortex excitability, we examined motor evoked potential, RMT, CSP, SICI and ICF. We determined RMT over the primary motor cortex in both groups by finding the minimal intensity required to elicit at least 5 motor evoked potentials of 50 mV out of 10 stimulations of the contralateral abductor digiti minimi muscle.
We obtained CSP by applying stimuli with an intensity of 40% above the active motor threshold. We applied and recorded TMS pulses with 10 trials at 140% of the active motor threshold during a low-level voluntary contraction of the abductor digiti minimi muscle. The duration of the CSP was defined in the rectified single trials as being from the end of the preceding motor evoked responses to the return of the amplitude of the mean voluntary electromyographic activity before TMS. The durations of each CSP elicited from 10 consecutive electromyographic signals were rectified and then averaged.
We used paired-pulse TMS with subthreshold conditioning to test SICI with ISIs of 2 ms and 3 ms and ICF with ISIs of 10 ms and 15 ms. The conditioning stimulus was set at 80% of the RMT and preceded the test stimulus (110% to 120% of the RMT), which produced a response of about 1 mV peak-to-peak amplitude. We applied 10 paired-pulse TMS trials of each ISI in 4 randomly intermixed conditions. Time between trials was 5 s. We measured the peak-to-peak amplitudes and then averaged them.
Measures of clinical symptoms and traits
We assessed all participants for obsessive–compulsive symptoms using the Maudsley Obsessional Compulsive Inventory (MOCI).24 The MOCI is a self-rating instrument of 30 dichotomous items designed to measure obsession and compulsion symptoms. It consists of 4 subscales: checking, washing, doubting and slowness. We also assessed patients with OCD using the Yale–Brown Obsessive Compulsive Scale (Y-BOCS)25 for the severity of obsessive–compulsive symptoms. We assessed depressive symptoms and anxiety levels using the Montgomery–Åsberg Depression Rating Scale (MADRS)26 and the Hamilton Anxiety Rating Scale (HARS),27,28 the most widely used semi-structured assessment scales, administered by trained psychiatrists. Missing values below 5% were replaced by an expectation-maximization algorithm.
Go/no-go test
To measure motor response inhibition, we performed the computerized go/no-go test (a faster variant of the classical go/no-go paradigm) in patients with OCD.5 We have previously reported impaired response inhibition in patients with OCD compared with healthy controls, using the go/no-go test.29 The task requires selection of a response (indicated by a “go” signal) or no response (indicated by a “no-go” signal). Patients with OCD were asked to respond to go signals (air-planes) appearing on the centre of the screen but not to no-go signals (bombs). The task was administered in 2 blocks, with 90 practice trials and 180 testing trials (126 go trials and 54 no-go trials) in randomized order. Patients were asked to inhibit their motor response when the no-go signals appeared on the screen. The ISI was 1000 ms, including a stimulus duration of 200 ms followed by a blank screen for 800 ms. The dependent variables of the inhibitory process were the percentage of successful inhibition trials and the mean reaction time for the correct go trials (ms).
Statistical analysis
We performed a priori power analysis using G*Power 3.1.23 A sample size of 68 participants (minimum 34 in each group) was needed to potentially detect a global effect of multivariate analysis of variance with a medium effect size (f2 V = 0.25), a power of 0.9 and 4 response variables.
We analyzed data using SPSS 23.0 for Windows (SPSS Inc.). Significance was set at p < 0.05. All tests were 2-tailed. We used the t test to evaluate differences in demographic and clinical characteristics between groups for continuous variables and χ2 tests for categorical variables.
We performed repeated-measures multivariate analysis of covariance (MANCOVA) to compare neurophysiological indices of TMS between patients with OCD and healthy controls, with hemisphere (left v. right) as the repeated factor, group (OCD v. controls) as the between-subjects factor, and TMS indices of RMT, CSP, SICI and ICF as the within-subjects factors. In MANCOVA, we used the mean values of the conditioned motor evoked potential size (a ratio of the conditioned motor evoked potential amplitude to the control motor evoked response) at ISIs of 2 ms and 3 ms as the parameter for SICI, and 10 ms and 15 ms for ICF. Covariates included age30 and sex31 to remove any possible effects on cortical excitability, based on previous findings. Significant results from MANCOVA were followed by separate univariate ANCOVA. We calculated ηp 2 values as a measurement of effect size, considering that a ηp 2 of 0.01 was small, 0.04 moderate and 0.1 large.32 For post-hoc ANCOVA, we adjusted the significance threshold using a Bonferroni approach to correct for tests of 4 dependent variables (i.e., 0.05/4 = 0.0125).
We also used partial correlations (pr) with covariates to explore the relationships between neurophysiological indices and clinical variables, such as onset age and parameter, regarding the inhibitory function on the go/no-go test (mean reaction time on the correct go trials) in patients with OCD.
Results
We found no significant differences between patients with OCD and controls in terms of age, sex or educational level. All patients were undergoing treatment with serotonin reuptake inhibitors (SRIs). Clinical characteristics are presented in Table 1.
Demographic and clinical characteristics between patients with OCD and healthy controls*
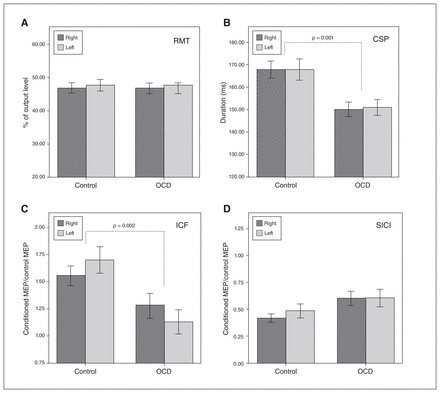
Repeated-measures MANCOVA with hemisphere (left v. right) as the repeated factor, diagnostic status of OCD as a between-subjects factor, age and sex as covariates, and TMS variables (RMT, CSP, ICF and SICI) as the within-subjects factors showed a significant difference between patients with OCD and healthy controls (F4,83 = 10.66; p < 0.001; ηp 2 = 0.339). We found no significant main effect of hemisphere (left v. right; F4,83 = 1.140; p = 0.34). We also observed no significant interaction effects between age and hemisphere (F4,83 = 0.555; p = 0.70), sex and hemisphere (F4,83 = 1.542; p = 0.20) or OCD diagnosis and hemisphere (F4,83 = 0.722; p = 0.58). In post hoc between-group comparisons, patients with OCD showed significantly shortened CSP compared to healthy controls (F1 = 12.604; p = 0.001; ηp 2 = 0.128; Fig. 1). Patients with OCD also had significantly decreased ICF (F1 = 10.298; p = 0.002; ηp 2 = 0.107). We found no significant difference in SICI (F1 = 3.879; p = 0.052; ηp 2 = 0.043) or RMT (F1 = 0.073; p = 0.79) between groups (Fig. 1).
Motor cortical excitability between patients with OCD and healthy controls. Graph showing variable means of bilateral RMT, CSP, ICF and SICI amplitudes in patients with OCD (n = 51) and controls (n = 39). Error bars represent ± 1 standard error of the mean. CSP = cortical silent period; ICF = intracortical facilitation; MEP = motor evoked potential; OCD = obsessive–compulsive disorder; RMT = resting motor threshold; SICI = short-interval intracortical inhibition.
After excluding patients with comorbid depression (n = 21), MANCOVA with the OCD group (n = 30) also showed that the between-group effects of CSP (F = 5.678; p = 0.020; ηp 2 = 0.080) and ICF (F = 11.798; p = 0.001; ηp 2 = 0.154) were still meaningful.
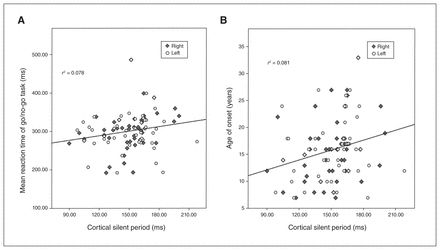
In the partial correlation analyses with age and sex as covariates, we found positive correlations at the trend level between CSP and mean reaction time in the go/no-go test (pr = 0.280; p = 0.051) and age of onset (pr = 0.285; p = 0.047; Fig. 2). When we included the MADRS score as a further covariate in partial correlations (because depressive symptoms may affect performance in the go/no-go task), the correlation between CSP and mean reaction time was significant (pr = 0.358; p = 0.013). On the other hand, we found no significant correlations between other neurophysiological indices of TMS and clinical symptom scores of OCD as measured by MOCI, Y-BOCS, MADRS or HARS.
Correlation between CSP and mean reaction time for the correct go trials in the go/no-go task and age at onset in patients with OCD (n = 51). CSP = cortical silent period; OCD = obsessive–compulsive disorder.
Discussion
The present study examined cortical excitability using paired-pulse TMS in patients with OCD and healthy controls to elucidate cortical inhibitory deficits in OCD. To our knowledge, it involves the largest sample of patients with OCD among studies using the paired-pulse TMS paradigm. Our results show that compared with controls, patients with OCD had altered cortical excitability in terms of shortened CSP and decreased ICF. We found no significant differences in RMT and SICI between patients with OCD and controls. These findings suggest that alterations of inhibitory neurotransmission mediated by GABA-B receptors and excitatory neurotransmission mediated by NMDA receptors may be involved in the pathophysiology of OCD.
Our finding of shortened CSP, a major inhibitory index, in OCD was consistent with previous results.19,21,33 Furthermore, in our correlation analyses, shortened CSP was associated with prompt reaction time in a go/no-go task and early onset of OCD. In particular, after adjusting for depressive symptoms, the correlation between CSP and reaction time in the go/no-go task was significant (p = 0.013). Shortened CSP in patients with OCD along with a shorter reaction time in go trials may indicate that the neurophysiological mechanism of impaired cortical inhibition plays a crucial role in impaired inhibitory control of thoughts and behaviours in OCD. In addition, because CSP at high stimulus intensity is considered a marker of GABA-B receptor-mediated inhibitory function,34 these findings in patients with OCD suggest that a lack of cortical inhibition via GABA-B receptor-mediated dysregulation might contribute to response inhibition and acceleration of OCD onset. However, since the present study had a cross-sectional design, further prospective research is needed to prove a possible association for OCD onset and response inhibition.
Regarding SICI, another main inhibitory parameter of TMS, our results did not show any significant finding of reduced cortical inhibition in patients with OCD compared with controls. This finding was consistent with that of Richter and colleagues,21 who found no differences between patients with OCD (n = 34) and healthy controls. However, it was inconsistent with a recent finding by Khedr and colleagues,33 which showed that patients with OCD (n = 45) had significantly reduced SICI compared with healthy controls. Greenberg and colleagues19 also showed that SICI in patients with OCD (n = 16) was significantly lower than in healthy controls. These discrepancies may have been partially due to the low statistical power of small sample sizes and medication effects. Since SRIs are known to modulate GABA release35 and enhance SICI,36 the possible effect of SRIs could have concealed any potential SICI deficits in the current study. To confirm the characteristics of cortical excitability in OCD, replication by future studies using paired-pulse TMS in larger samples with drug-naive patients is needed.
Contrary to our expectations, for the facilitatory component of intracortical excitability, patients with OCD had significantly decreased ICF compared with healthy controls, indicating reduced glutamatergic signalling in OCD. The effect size of decreased ICF was greater after excluding patients with comorbid depression (ηp 2 = 0.154). Conversely, Greenberg and colleagues19 reported higher mean values for ICF in OCD than in controls, although this difference did not reach significance. Richter and colleagues21 also showed that patients with OCD had greater ICF than healthy controls. As well, mixed findings have been observed for levels of cortical glutamate transmission in patients with OCD.37,38 These inconsistent findings may be partially explained by the clinical heterogeneity of OCD, medication effects, limited statistical power and the different phase of treatment courses across studies. Although the findings remain inconclusive, the altered ICF observed in patients with OCD might reflect a disrupted neuroplasticity via altered glutamate-mediated excitatory neurotransmission in OCD. Evidence from TMS studies in depressive patients suggests that reduced facilitation and impaired glutamate-mediated neuroplasticity in response to paired associative stimulation are implicated in depression and cognitive dysfunction.39,40 Further research is required to confirm the present findings and to gain a better understanding of alterations in cortical excitability and neuroplasticity in patients with OCD.
Together, our findings of altered CSP and ICF provide further neurophysiological evidence for an imbalance in inhibitory and excitatory function in the cortical circuits of patients with OCD. This imbalance is also supported by substantial evidence from functional imaging studies, which have shown the involvement of CSTC circuits in OCD.4,41,42 Within the CSTC circuitry, the interactive loop between the GABAergic inhibitory and glutamatergic excitatory pathways is thought to be responsible for balancing neural tone.4,38 Genetic studies have also shown the involvement of genes related to GABA or glutamate in OCD pathogenesis.43–45 The imbalance of GABAergic and glutamatergic receptor–mediated neurotransmission in CSTC circuitry may contribute to the regional hyperactivity and lack of response inhibition seen in OCD, leading to obsessive–compulsive symptoms.
Limitations
Several limitations should be mentioned. First, although there was no difference in the degree of SICI between medicated and unmedicated patients in previous studies,19,33 we cannot exclude the influence of medications on motor cortex excitability. All patients in the present study were undergoing SRI treatment, and some were on concomitant benzodiazepines or antipsychotics. Alterations in TMS measures produced by medication have been reported to be complex depending on the individual drug and chronic use: a single dose of citalopram enhances CSP and SICI, whereas chronic paroxetine use does not alter either CSP or SICI but enhances ICF.46 In addition, benzodiazepines enhance SICI and reduce ICF, whereas dopamine antagonists, such as antipsychotics, increase ICF.47 For paired-pulse TMS measurements, medications influencing GABA, glutamate, serotonin or dopamine neurotransmission35 could affect GABAergic and glutamatergic receptor-mediated neurotransmission in CSTC circuitry and neuroplasticity.47,48 Second, the limited sample size may make it difficult to draw definitive conclusions, because low statistical power may lead to increased rates of false negatives and false positives. Third, since a role for the GABAergic and glutamatergic system has also been reported in the pathophysiology of depression, comorbid depression may have biased the present results. Previous TMS studies have shown that patients with depression had reduced excitability of both inhibitory and facilitatory inputs compared with controls.49,50 However, our results of shortened CSP and decreased ICF still remained significant after excluding individuals with comorbid depression. The correlation between CSP and response inhibition on the go/no-go task was also significant after adjusting for depressive symptoms. Therefore, we believe that the findings of altered cortical excitability may be specifically related to OCD, rather than depression. Fourth, this study is limited by the lack of neuroradiological imaging and of estimates of peripheral nerve excitability and central motor conductivity. Finally, we examined TMS indices of cortical excitability only in the motor cortex, which may not be relevant to key brain regions of OCD. Measurements of cortical excitability in brain regions critically involved in OCD may be helpful for understanding the underlying pathophysiology of OCD.
Conclusion
The present study showed that patients with OCD had altered cortical excitability with shortened CSP and decreased ICF. In addition, the associations between CSP and response inhibition and onset age further suggest that cortical inhibition may be involved in the pathophysiology of OCD. Our findings support the role of altered cortical excitability in contributing to symptom formation in OCD. Further research in larger samples that include unmedicated patients is warranted to elucidate the pathophysiology of OCD.
Acknowledgements
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2015R1D1A1A09058829).
Footnotes
Competing interests: None declared.
Contributors: J.I. Kang, D.Y. Kim and S.J. Kim designed the study. All authors acquired the data, which J.I. Kang and S.J. Kim analyzed. J.I. Kang wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
- Received April 24, 2018.
- Revision received August 6, 2018.
- Accepted September 6, 2018.