Abstract
Background: Emotion dysfunction is a key symptom in patients with borderline personality disorder (BPD) and is considered a consequence of dysfunctional emotion regulation (e.g., reduced emotion acceptance). In the present functional MRI (fMRI) study, we investigated the neural correlates of habitual emotion acceptance in individuals with BPD.
Methods: Female patients with BPD and female healthy controls passively viewed negative and neutral movie clips of faces during fMRI. We assessed emotion acceptance using the Emotion Acceptance Questionnaire (EAQ). To examine brain activation associated with habitual emotional acceptance of negative stimuli, the EAQ score was included as a regressor of interest in brain data analyses of activation intensity during negative compared with neutral movies.
Results: We included 20 women with BPD and 20 heatlhy controls in our analysis. Compared with healthy controls, patients with BPD showed significantly more activation in frontostriatal brain regions (i.e., left superior frontal gyrus, right caudate) as well as in the left precuneus, left precentral gyrus, left posterior cingulate cortex and left hippocampus when confronted with negative (v. neutral) stimuli. Patients with BPD reported decreased emotion acceptance compared with healthy controls, and habitual emotion acceptance was inversely associated with activation of striatal areas (i.e., left putamen, left caudate) in patients with BPD.
Limitations: Causal conclusions are not possible. Comorbid diagnoses were not excluded, and only female participants were investigated. Stimuli were not rated immediately and may not be generalizable to all negative emotions. We cannot make any statements about other emotion-regulation strategies that may have been applied here.
Conclusion: Data indicate that striatal hyperactivation during the processing of negative stimuli in women with BPD is related to their decreased disposition to accept unpleasant emotional states. Thus, individuals with BPD may benefit from therapy approaches that focus on emotion acceptance in order to normalize emotional reactions.
Introduction
Emotion dysfunction is the primary symptom of patients with borderline personality disorder (BPD)1 and affects the occurrence and maintenance of further BPD symptoms.2,3 Emotion dysfunction is considered a consequence of impaired emotion regulation.4–7 In the context of emotion dysregulation, patients with BPD report a high rate of emotion suppression and reduced emotion acceptance.4,8,9 Gross10 related suppression to the expression of emotions. However, other authors define emotion suppression as a more general attempt to reduce the entire emotional reaction, including psychological and psychophysiological characteristics.11
Emotion suppression is often considered a counterpart of emotion acceptance.12–14 Emotion acceptance means an openness to emotions as they unfold.15 Paradoxically, emotion suppression has been found to correlate with stronger and longer-lasting experience of negative emotional states and physiologic arousal.16 On the other hand, emotion acceptance has increasingly attracted attention as an adaptive way to deal with emotional arousal.17 In nonclinical populations, acceptance appeared to be more effective than no regulation or suppression, resulting in better cognitive performance and enhanced mood18 as well as less physiologic arousal after viewing distressing pictures.19 With regard to clinical populations, enhanced emotion acceptance is related to reduced psychopathology.20 Compared with emotion suppression, for example, Campbell-Sills and colleagues16 found positive effects of emotion acceptance on negative affectivity in clinical samples with anxiety or mood disorders. Respective investigations involving patients with BPD are uncommon. Being one of the few studies targeting emotion acceptance in BPD, Gratz and Gunderson5 found positive outcomes of an acceptance-based emotion regulation intervention on overall symptoms (e.g., negative affectivity). Another study reported a positive effect on psychological strain in BPD after 10 weeks of mindfulness training including acceptance skills.21
In accordance with these findings, functional MRI (fMRI) studies indicate divergent emotion-related neural activity in BPD. Compared with healthy individuals, patients with BPD show alterations of neural activity in prefrontal brain areas such as the dorsolateral prefrontal cortex (DLPFC) or the anterior cingulate cortex (ACC) during the processing of negative emotions and a hyperactivation of (para-)limbic regions, particularly the amygdala.22,23 Moreover, functional and structural abnormalities of the hippocampus in connection with emotional memory and experience have been reported in patients with BPD.22,24 In a passive viewing paradigm of emotionally aversive pictures compared with neutral pictures, Herpertz and colleagues25 and Hazlett and colleagues26 found increased activation of medial and inferior frontal gyri, the fusiform gyrus and the amygdala in patients with BPD compared with healthy controls. In another study that compared neural activity while viewing negative pictures compared with a resting state, individuals with BPD showed higher activation in the amygdala, fusiform and superior temporal gyrus, and in visual and premotor areas.27 Two previous studies by our working group found frontolimbic hyperactivation in patients with BPD, mainly in the amygdala, ventral and dorsal cingulate cortex, and frontotemporal areas including the anterior and inferior frontal gyrus and anterior temporal and mediotemporal areas, associated with the recall of emotionally negative life events.28,29 Moreover, Winter and colleagues30 investigated neural activity of patients with BPD before and 12 weeks after dialectical behaviour therapy7 (DBT). Responders to DBT exhibited decreased right perigenual anterior cingulate activity when viewing negative compared with neutral pictures.
With respect to emotion regulation, researchers have instructed participants to apply emotion-regulation strategies under fMRI conditions. Schulze and colleagues,31 for example, reported less activation of the left orbitofrontal cortex in patients with BPD than in healthy controls when participants were asked to downregulate their emotional response to aversive pictures. By contrast, their activation within the bilateral insula was increased. Koenigsberg and colleagues32 investigated the ability of patients with BPD to distance themselves from negative pictures. Compared with healthy individuals, patients with BPD showed less activation of the dorsal ACC and parietal brain regions (contrast looking v. distancing from negative pictures). They also showed less deactivation of the amygdala as well as hyperactivation of the superior temporal sulcus and the superior frontal gyrus. Moreover, a recent study involving patients with BPD focused on neural activity before and 12 weeks after DBT.33 In particular, responders to DBT showed reduced activity in the amygdala, ACC, orbitofrontal cortex and DLPFC after DBT during a reappraisal task of negative stimuli. In all of these studies, participants were instructed to apply certain emotion regulation strategies so the researchers could examine whether patients implemented those strategies in the same way as healthy people or whether they implemented them more successfully after treatment.
Goodman and colleagues34 chose a different approach to investigate neural correlates of emotion regulation. Patients with BPD were assessed before and after 12 months of DBT. Compared with untreated healthy controls, patients with BPD showed a posttreatment reduction of amygdala-related fMRI activity when watching emotionally relevant or irrelevant pictures. In part, improvement of emotion-regulation skills including emotion acceptance was related to a reduction of amygdala activity. Because a main component of DBT addresses the acceptance of feelings, this result can be interpreted as a positive effect of DBT. However, emotion regulation and neural activity are not directly related in this study design.
Consequently, to our knowledge, there are no studies involving patients with BPD that directly target neural correlates of habitually used emotion regulation in general and emotion acceptance in particular. Therefore, the aim of our study was to investigate the neural correlates of habitually used (uninstructed) emotion acceptance in BPD. Generally, we hypothesized there would be activation changes of the frontolimbic emotion regulation network in the BPD group in response to emotionally negative stimuli. Based on previous findings, we particularly expected increased amygdala and insula activation in patients with BPD compared with healthy controls during passive viewing of negative stimuli. With respect to the DLPFC, ACC and hippocampus, previous results regarding hypo- or hyperactivation are inconsistent, which does not allow directed hypotheses. With respect to the main focus of this study, we additionally assumed that reduced acceptance of negative emotions in individuals with BPD would be associated with respective activation changes in the emotion-regulation network.
Methods
Participants
We recruited women with BPD and female healthy controls matched for age and education to participate in our study. The BPD group was recruited at the Clinic of Psychiatry and Psychotherapy, Evangelisches Klinikum Bethel, Germany. Healthy controls were recruited via local advertisements. For all participants, exclusion criteria were current or previous severe medical conditions (e.g., malignant tumour) or neurologic disorders (e.g., epilepsy) as well as conditions incompatible with MRI investigations (e.g., pacemaker, pregnancy). Moreover, individuals with any kind of current or anamnestic psychiatric disorder were not eligible to participate in the control group. Psychotropic medication was not allowed. Additional exclusion criteria for patients with BPD were the intake of benzodiazepines, changes of psychotropic medication within the last 14 days, severe comorbid mental disorders (e.g., affective disorder, substance dependence or anorexia nervosa) that were not fully remitted for at least 6 months as well as DBT treatment within the last 6 months. Patients with schizophrenia were entirely excluded.
All participants underwent a psychological diagnostic interview. Diagnoses (patients) and the exclusion of diagnoses (healthy control participants) were based on the German version of the Structural Clinical Interview for DSM-IV (SCID-I and SCID-II) and the BPD section of the SCID-II,35 administered by experienced and trained clinical psychologists. Symptoms were assessed using the Borderline Symptom List36 (short version; BSL-23).
Ethical approval for the study was provided by the Ethics Committee of the University of Muenster, Germany. Prior to study participation, all participants had given their written informed consent. Participants received compensation of €50.
Experimental procedure
To investigate the neural correlates of the emotional response to negative stimuli, we administered a modified version of the fearful face paradigm during fMRI37 with validated movie sequences showing 96 different male and female fearful (negative condition) or neutral (neutral condition) faces. The paradigm originally included movie sequences of fearful faces as the experimental condition and landscapes as the control condition. To increase the specificity of this contrast, we modified the paradigm by substituting the control condition showing landscapes with a condition showing neutral faces. This modification allowed us to link effects directly to the valence of the presented faces while controlling for effects associated with movements or face recognition. Participants were instructed to watch the movie sequences passively.
Each of the experimental trials can be subdivided into 3 phases: introduction, stimulus presentation (movie sequences) and pause (Appendix 1, Fig. S1, available at jpn.ca/180077-a1). During the introduction phase, the words “Los geht’s” (English: “Here we go”) were displayed in white letters on a black screen, indicating the beginning of each trial. Duration of the introduction phase was 3200 ms on average with a variable jitter between 1700 ms and 4700 ms.38 After this introduction, movie sequences were presented for a time interval of 16000 ms. In these movie sequences, either negative or neutral faces were shown in pseudorandomized order. Each condition of movie sequences contained 6 different faces. After the movie sequence, the word “Pause” was presented in white letters on a black screen for 1700 ms. During this pause, a white fixation cross on a black background was presented for another 16 000 ms. Altogether, participants had to perform 6 experimental trials per condition and 2 partial trials per condition (introduction and movie sequence only). Partial trials were implemented to allow for the isolation of blood-oxygen level–dependent (BOLD) signal changes to the different phases of the task.39 The total duration of the experimental conditions was about 9 min. Before and after the fMRI experiment, participants rated their current affective state using the Positive and Negative Affect Schedule40 (PANAS).
Clinical measures
To relate brain activity to habitual emotion acceptance, we used the German Version of the Emotion Acceptance Questionnaire8 (EAQ). The EAQ shows excellent psychometric properties with a Cronbach α between 0.86 and 0.89 and a convergent validity with the Difficulties in Emotion Regulation Scale41 (DERS) of r = −0.43 to r = −0.65 in clinical and nonclinical populations.8,42 The EAQ provides 2 main scales: acceptance of positive and negative emotions. As we investigated negative emotions here, we included only the acceptance of negative emotions scale, comprising 16 items. In our sample the Cronbach α was 0.93 for the healthy controls and 0.84 for the BPD group. The PANAS,40 administered to assess negative affectivity, includes 10 items that have to be rated on a 5-point Likert scale.
Imaging data acquisition
Brain data were acquired in the MARA epilepsy centre (Bielefeld, Germany) using a 3 T Siemens Magnetom Verio whole-body scanner (Siemens) with a quantum gradient and a standard 12-channel head coil. Functional images were obtained using a single T2-weighted gradient echo planar imaging (EPI) sequence with the following parameters: slice thickness 4 mm (1 mm gap), repetition time (TR) 2100 ms, echo time (TE) 30 ms, flip angle 90°, field of view (FOV) 192 × 192 mm, matrix size 64 × 64, voxel size 3 × 3 × 4 mm. The number of volumes was 377, each containing 30 axial slices covering the whole brain and measured in descending order parallel to the hippocampus. During scanning, participants lay comfortably in a supine position in the scanner. An adjustable head holder restricted potential head movements. Visual stimuli were displayed on a back-projection screen (1024 × 768 pixels) near the tube end using Presentation software (Neurobehavioural Systems). Participants watched the screen via a dual-mirror that was mounted to the head coil. Before the EPI sequence, field map sequences were applied to control for magnetic field inhomogeneities. After the EPI sequence, high-resolution anatomic images were acquired using a T1-weighted, 3-dimensional magnetization-prepared rapid gradient echo (MPRAGE) sequence (slice thickness 0.8 mm, TR 1900 ms, TE 2.5 ms, inversion time 900 ms, flip angle 9°, FOV 240 × 240 mm, matrix size 320 × 320, voxel size 075 × 0.75 × 0.75 mm, 192 slices).
Statistical analysis
Behavioural data
Behavioural data analysis of the PANAS included a 2 × 2 (group × time) repeated-measures analysis of variance (ANOVA). The between-subjects factor was experimental group (BPD v. healthy controls) and the within-subjects factor was time of assessment (before v. after the movie sequences). For the EAQ, 2-sample t tests were conducted. Data were analyzed using IBM SPSS Statistics version 20.0 (SPSS Inc.). All levels of significance were α < 0.05 and 2-tailed.
Imaging data
Imaging data were analyzed using SPM8 (Wellcome Department of Cognitive Neurology; http://www.fil.ucl.ac.uk/spm). The first 3 images of every EPI recording session were discarded to account for the time needed for the magnetic field to achieve a steady state. The EPI data were preprocessed, including movement and slice time correction, coregistration to the individuals’ structural images, 12 parameter nonlinear normalization (3 × 3 × 3 mm3) into the Montreal Neurological Institute (MNI) reference space, and smoothing with an isotropic 3-dimensional Gaussian kernel with a full-width at half-maximum (FWHM) of 9 mm. Functional imaging data were analyzed using a general linear model with 5 regressors, including 1 for the movie sequences showing negative faces, 1 for the movie sequences showing neutral faces, 2 for the fixation cross durations after neutral and negative movies, and 1 combined regressor for the introductions. In addition, the 6 alignment parameters were included into the design matrix as movement regressors of no interest. Regressors were convolved using the hemodynamic response function as provided in SPM 8. Design matrices were high-pass filtered (128 s).
To identify neural activation associated with negative emotions, activation during the movie sequences showing fearful faces was contrasted with activation during the sequences showing neutral faces. This contrast (negative – neutral) was computed for each participant on the first level. To identify neural networks associated with negative emotions in each group as well as between these groups, contrast images were then applied to second-level random-effects within- and between-group analyses. To examine the impact of the level of habitual emotion acceptance on brain activation in the single experimental groups, we included the individual EAQ values (total score for acceptance of negative emotions) as a regressor of interest into the within-group analyses and examined variance explained by this regressor.
All analyses were performed at the whole-brain level. Whole-brain results were tested at the cluster level using a threshold of Z > 3.1 with a minimum cluster size of k ≥ 20 voxels and a cluster significance threshold of p < 0.05, family-wise error (FWE)-corrected for multiple comparisons.
Moreover, we used a region-of-interest (ROI) approach to examine the neural response to negative emotions in brain regions known to be associated with emotion regulation. The ROIs were determined a priori based on previous findings and included the amygdala, insula, hippocampus, ACC and DLPFC.43–45 The corresponding ROI masks were taken from the automated anatomic labelling atlas46 (AAL), which is implemented in the Wake Forest University (WFU) PickAtlas, an automated software toolbox for generating ROI masks based on the Talairach Daemon database.47–50 The ROIs of the DLPFC were defined as the aggregation of Brodmann areas (BAs) 9 and 46 (also implemented in the WFU PickAtlas). For each ROI, hemisphere and participant, contrast values from the negative – neutral contrast were extracted using the SPM Marsbar toolbox.51 These contrast values represented BOLD signal changes from the neutral to the negative condition. Individual contrast values were then fed into a 5 × 2 × 2 (ROI × hemisphere × group) repeated-measures ANOVA using IBM SPSS Statistics 20.0 (SPSS Inc.). Moreover, exploratory Pearson correlations between ROI-specific signal change and the EAQ score were calculated for both groups separately.
Results
Participants
In total, 45 patients with BPD were informed about the study. Twenty-five had to be excluded from participation owing to severe medical conditions, left-handedness or a lack of interest in participation, leaving a final sample of 20 women with BPD and 20 matched controls for analysis. Characteristics of the study group are reported in Table 1. All participants were right-handed with normal or corrected-to-normal vision. On average, patients with BPD met 6.7 ± 1.13 out of 9 BPD criteria. Accordingly, the BSL-23 showed more symptoms in the BPD group than in the healthy control group. Twelve patients with BPD were treated with psychotropic medication (antidepressants: n = 12, neuroleptics: n = 9). However, analyses revealed no differences between patients with and without medication. In 9 patients, BPD was the only diagnosis. Nine patients had 1 comorbid mental disorder and 2 patients had 2 comorbid disorders. Posttraumatic stress disorder (n = 6) was the most common comorbid diagnosis. A complete list of comorbidities can be found in Appendix 1, Table S1.
Demographic and clinical characteristics of the study sample
Behavioural data: habitual emotion acceptance
Compared with healthy controls, patients with BPD showed significantly lower mean scores of emotion acceptance (Table 1). For the PANAS, repeated-measures ANOVA revealed a significant main effect of time (F1,38 = 4.21, p = 0.047, η2p = 0.100) and a significant main effect of group (F1,38 = 13.44, p = 0.001, η2p = = 0.261) but no time × group interaction effect (F1,38 = 1.81, p = 0.186). These results indicate that negative affectivity was reduced over time in both groups and that individuals with BPD showed generally more negative affect than healthy controls.
Functional MRI data
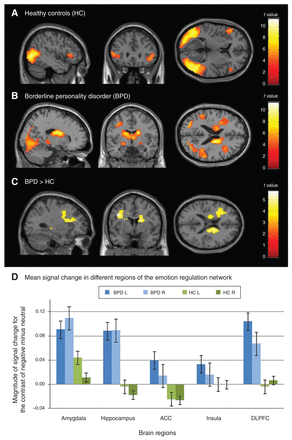
In healthy controls, whole-brain analysis showed increased posterior and frontal activation in the negative compared with the neutral condition (negative – neutral) (Table 2). Whole-brain analysis identified 4 main clusters with activation peaks in the bilateral middle occipital and bilateral inferior frontal gyri (Fig. 1A). Moreover, these clusters included the left fusiform gyrus and the right middle temporal gyrus. In the BPD group, whole-brain analysis identified 1 large cluster with activation peaks in the right middle temporal gyrus, the right fusiform gyrus and the left caudate (Fig. 1B). Between-group comparisons showed that patients with BPD showed more activation associated with negative emotions (negative – neutral contrast) than healthy controls (Fig. 1C). Whole-brain analysis identified 2 clusters with activation peaks in the left precentral gyrus and the left precuneus. Moreover, these clusters included the left superior frontal gyrus, right caudate, left hippocampus and left posterior cingulate cortex (PCC) (Table 2). The opposite comparison (HC > BPD) did not reveal significant effects. Notably, the 2 groups did not differ with respect to activation intensity in the neutral condition.
Brain activation associated with negative emotions (negative – neutral contrast) in (A) healthy controls and (B) patients with BPD as well as (C) increased brain activation related to negative emotions in patients with BPD compared with healthy controls and (D) mean signal changes in different regions of the emotion-regulation network for both groups and hemispheres separately, displayed together with standard errors of the means. Displayed are the whole-brain results of within-group and between-group cluster-level analyses with cluster significance thresholds of p < 0.05, FWE-corrected for multiple comparisons, and minimum cluster sizes of 20 voxels (A–C) and repeated-measures analysis of variance of regions of interest (D). ACC = anterior cingulate cortex; BPD = borderline personality disorder; DLPFC: dorsolateral prefrontal cortex; FWE = family-wise error; HC = healthy controls; L = left hemisphere; R = right hemisphere. Mean signal change in different regions of the emotion regulation network Brain regions Magnitude of signal change for the contrast of negative minus neutral
Brain activation associated with negative emotions in patients with BPD and healthy controls as well as significant differences between both groups for the negative – neutral contrast*
To identify neural activity associated with negative emotions within the emotion regulation network (amygdala, hippocampus, ACC, insula and DLPFC), we used an ROI approach. Results of the 5 × 2 × 2 (ROI × hemisphere × group) repeated-measures ANOVA showed a significant main effect of group (F1,38 = 6.95, p = 0.012, η2p = 0.155), indicating higher activation in patients with BPD than in healthy controls across all ROIs in both hemispheres (Fig. 1D). Other main and interaction effects did not reach statistical significance. The ANOVA results suggest that patients with BPD, compared with healthy controls, showed a more pronounced neural response of the whole emotion regulation network when being confronted with negative stimuli.
Association between habitual emotion acceptance and fMRI data
To relate our findings to habitual emotion acceptance, we included the individual EAQ score (total score for acceptance of negative emotions) as a regressior of interest into the within-group analyses of activation associated with negative emotions (negative – neutral contrast) for both groups separately. In healthy controls, whole-brain analysis did not reveal significant effects. For the BPD group, however, lower levels of emotion acceptance correlated with higher activation of the left dorsal striatum (Fig. 2). Whole-brain analysis identified 1 large cluster with activation peaks in the left putamen and the left caudate (Table 2).
Top: negative correlation between fear-related activation (negative – neutral contrast) and the level of habitual acceptance (EAQ) in patients with BPD. Displayed are the whole-brain results of a within-group cluster-level analysis including the EAQ score as a regressor of interest with a cluster significance threshold of p < 0.05, FWE-corrected for multiple comparisons, with a minimum cluster size of 20 voxels. Bottom: correlation of signal change in the left dorsal striatum (negative – neutral contrast) and acceptance of negative emotions (EAQ) in patients with BPD. BPD = borderline personality disorder; EAQ = Emotion Acceptance Questionnaire; FWE = family-wise error. Acceptance of negative emotions (EAQ) Magnitude of signal change for the negative – neutral contrast
Moreover, exploratory analyses showed a trend toward significant negative correlations of the EAQ score with left (r = −0.40, p = 0.081) and right (r = −0.39, p = 0.09) DLPFC activity in the BPD group, indicating tendentially decreasing bilateral DLPFC activation with increasing acceptance. In the group of healthy controls, we found a trend toward significant positive correlations of the EAQ score with left (r = 0.39, p = 0.09) and right (r = 0.43, p = 0.06) ACC activity and a trend toward significant negative correlations of the EAQ score with activation intensity of the right hippocampus (r = −0.38, p = 0.09). The latter results suggest tendentially increasing ACC and decreasing hippocampal activation intensity with increasing acceptance in healthy controls.
Discussion
In the present work, we investigated the neural correlates of habitually applied (uninstructed) emotion acceptance in women with BPD. To our knowledge, this is the first fMRI study on this topic. Compared with healthy controls, patients with BPD showed hyperactivation of frontostriatal, PCC, hippocampal and posterior parietal (precuneus) brain areas when confronted with negative (v. neutral) stimuli. In addition, activation of striatal areas was inversely related to habitual emotion acceptance. As individuals with BPD also reported decreased emotion acceptance compared with healthy controls, our data indicate that striatal hyperactivation during the processing of negative stimuli in patients with BPD is associated with their decreased disposition to accept unpleasant emotional states. Furthermore, our findings revealed tendentially decreasing bilateral DLPFC activation with increasing acceptance in patients with BPD as well as tendentially increasing ACC and decreasing hippocampal activation intensity with increasing acceptance in healthy controls.
To date, most fMRI studies in this field have explored the effect of emotion acceptance as a facet of mindfulness. These studies found associations between mindfulness and patterns of neural deactivation in frontolimbic and temporal brain areas.52,53 According to a study of healthy volunteers by Farb and colleagues,54 mindfulness-based emotion regulation may particularly reduce activation of brain areas, such as the amygdala, that are related to emotional arousal. Confirming this assumption, an fMRI study by Lutz and colleagues55 investigated the effects of brief mindfulness training on the processing of negative emotions in healthy participants. Twenty-four participants received this training and were then instructed to focus mindfully on negative and neutral pictures. A control group with 22 healthy participants received no mindfulness training and they did not receive instructions on how to view the pictures. Compared with the control group, the mindfulness group showed less activation of the amygdala while viewing negative compared with neutral pictures. Contrary to our study, however, this investigation focused on mindfulness and not particularly on acceptance. In fact, apart from emotion acceptance, the practice of mindfulness also includes other functional emotion regulation strategies, such as attentional deployment and cognitive reappraisal.56,57 Thus, it seems plausible that mindfulness may have a broader impact on emotion-related brain areas than solely emotion acceptance.
As far as we can tell, the neural correlates of emotion acceptance itself were studied only by Smoski and colleagues.58 The authors investigated the instructed acceptance of sad images compared with passive viewing or reappraisal of sad images in healthy participants and patients with remitted depressive disorders. In both groups, participants showed a stronger activation of the left lateral PFC, the orbitofrontal cortex, the frontal pole and the ACC when accepting compared with passively watching sad images. The authors suggest that these acceptance-related PFC and ACC hyperactivations may reflect enhanced metacognitive processing in the sense of greater awareness of one’s own cognitive and emotional processes. In contrast to Smoski and colleagues,58 however, we did not instruct acceptance, but rather investigated the neural correlates of habitual emotion acceptance. As the realization of an instruction requires metacognitive processes, it may be assumed that the PFC and ACC activations of the participants in the study by Smoski and colleagues are related to the conscious and cognitively supervised realization of an instruction rather than to emotion acceptance itself.
Irrespective of the correspondence of our acceptance-related results with the results of other studies, the question of how we can explain the dorsostriatal (putamen and caudate) hyperactivation in individuals with BPD as a neural correlate of decreased emotion acceptance remains. In line with Gratz and Tull,59 emotion acceptance implies the tolerance of aversive emotions and, thus, may reduce the need for a behavioural reaction to distressing emotional experiences. Consequently, reduced emotion acceptance increases the need to react to the aversive emotional experience. On a neuronal level, the striatum links emotions and motion control by its connections to prefrontal and limbic brain areas.60 Whereas the putamen is primarily associated with the implementation of behaviour, activation of the caudate is crucial for the planning of goal-directed behaviour.61,62 In particular, when a positive (or less negative) outcome of a specific behaviour is anticipated, activation of the caudate is increased.63 Moreover, D’Argembeau and colleagues64 found enhanced activity in the caudate when participants anticipated near-future events. They interpreted this activation in the context of a “mental simulation” of forthcoming emotional events and actions with a positive outcome. Therefore, our finding of dorsostriatal hyperactivation can be interpreted as a neural correlate of a behavioural impulse or of cognitive-executive processes in response to a distressing and not accepted emotional experiences or both. The assumption of the emergence of a behavioural impulse in response to a distressing and not accepted emotional experience is supported by our finding that individuals with BPD also showed hyperactivation of the posterior part of the superior frontal gyrus and the precentral gyrus at negative compared with neutral stimuli. These brain areas are known to be associated with motion control.65 The assumption of increased emergence of cognitive-executive processes in response to a distressing and not accepted emotional experience is also supported by exploratory findings of our study. In the group of patients with BPD (but not in healthy controls), increasing activation of the DLPFC reflecting enhanced executive control was tendentially associated with decreasing emotion acceptance.
Apart from the main finding of our study, individuals with BPD also showed stronger activation than healthy controls when responding to negative compared with neutral movie scenes in a cluster that included the left PCC, left precuneus and left hippocampus, irrespective of emotion acceptance. the PCC and precuneus are parts of the default mode network with a close connection to the hippocampus, and they contribute to self-referential processes.66–68 In this context, our results can be interpreted as a neural correlate of stronger internally focused cognition in patients with BPD when confronted with negative emotions, which is in line with the results of studies that showed a higher self-relevance of emotional information in patients with BPD.69
Finally, we explored a frontolimbic emotion regulation network (including the amygdala, hippocampus, ACC, insula and DLPFC). In line with the findings of Krause-Utz and colleagues,22 we found greater activation in individuals with BPD than in healthy controls across all ROIs when watching negative compared with neutral movie scenes. In general, limbic hyperreactivity in BPD is interpreted as an increased preparedness for high sensitivity to emotional stimuli. Moreover, our results also revealed hyperactivation of the ACC and DLPFC, which is consistent with previous findings of our working group.28 At that time, we suggested that prefrontal hyperactivation may indicate increased but inefficient attempts to suppress rather than to accept emotions in individuals with BPD. This interpretation corresponds to the present and recent study findings8 of decreased emotion acceptance in BPD.
Limitations
Our study has both strengths and limitations. To our knowledge, this is the first fMRI study involving individuals with BPD that considers habitual emotion acceptance while processing emotional content. This seems to be of particular importance, as individuals with BPD show decreased emotion acceptance,8 and emotion acceptance is regarded as an important functional emotion-regulation strategy.16 In addition, we used stimuli (facial expressions) that differed only with respect to the emotional content, ensuring that confounding factors such as the processing of social or facial attributes were widely eliminated. Furthermore, results were not influenced by any kind of cognitive task or instruction, allowing the assessment of neural correlates of natural emotion processing in connection with habitual emotion acceptance.
However, we have correlated emotion acceptance with neural activity. As such, causal conclusions are not possible and we cannot exclude an influence of other variables (e.g., empathy) on our results. Second, some patients fulfilled the diagnostic criteria of additional disorders, such as PTSD. However, symptoms of comorbid disorders are typical in patients with BPD, and exclusion of all comorbid diagnoses would have led to a nonrepresentative patient group. Nevertheless, we cannot rule out that the results reported here may be related to comorbid disorders rather than to BPD per se. Third, medication may have influenced our results, but exclusion of medicated patients would have decreased the generalizability of our results. However, we did not find differences between patients with and without medication. Generalizabilty is also limited by the fact that only female participants were investigated in our study and that the sample size was quite small. Fourth, we did not decide to implement immediate emotional valence ratings after each stimulus presentation to prevent an impact of these ratings on neural activity.70 In fact, we wanted to ensure feasible duration and flow of our experiment. Fifth, we do not know which emotion-regulation strategies were applied. However, correlation between emotion acceptance and brain activity suggest that emotion acceptance is related to mental activity. We cannot make any statements about other emotion regulation strategies that may have been applied. Finally, as we used stimuli showing only fearful faces, our findings might not be generalizable to all negative emotions.
Conclusion
The investigation of functional brain imaging data associated with habitual emotion acceptance is meaningful for the understanding of emotion dysregulation and its neural correlates in BPD. Our results provide evidence of dorsostriatal hyperactivation in individuals with BPD during the processing of negative stimuli. This hyperactivation is associated with decreased disposition to accept negative emotions in individuals with BPD. Therefore, our study indicates that emotion acceptance may be a helpful emotion-regulation skill for patients with BPD to normalize their emotional reactions. The results support therapy approaches such as DBT7 that include the training of emotion acceptance. Further research needs to show whether such approaches lead to a normalization of emotion acceptance and its neural correlates in patients with BPD.
Acknowledgements
The authors thank Violetta Swiatek for her expertise and kind help during the fMRI investigations. The authors also thank all participants for their willingness to take part in this study. The authors thank the German Research Foundation (“Deutsche Forschungsgemeinschaft”, DFG) for the financial support.
Footnotes
Funding: This work was financially supported by the DFG (“Deutsche Forschungsgemeinschaft”, grants: BE 2536 / 9-1, TO 894 / 3-1).
Competing interests: None declared.
Contributors: M. Toepper, S. Carvalho Fernando, M. Driessen and T. Beblo designed the study. A. Lamers, N. Schlosser and F. Woermann acquired the data, which A. Lamers, M. Toepper, S. Carvalho Fernando, E. Bauer, M. Driessen and T. Beblo analyzed. A. Lamers and T. Beblo wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
- Received June 6, 2018.
- Revision received December 11, 2018.
- Accepted January 24, 2019.