Abstract
Background: Serotonergic system abnormalities are implicated in many psychiatric disorders, including major depression. The temporal lobe receives a high density of serotonergic afferent projections, and responses in the primary auditory cortex to sound are modulated by serotonergic tone. However, the associations between changes in serotonergic tone, disease state and changes in auditory cortical function remain to be clarified.
Methods: We quantified serotonin 1A (5-HT1A) receptor binding, serotonin 2A (5-HT2A) receptor binding, and serotonin transporter (SERT) binding in Brodmann areas (BA) 41/42, 22, 9 and 4 from postmortem brain sections of 40 psychiatrically healthy controls and 39 individuals who had a history of a major depressive episode (MDE).
Results: There was 33% lower 5-HT2A receptor binding in BA 41/42 in individuals who had an MDE than in controls (p = 0.0069). Neither 5-HT1A nor SERT binding in BA 41/42 differed between individuals who had an MDE and controls. We also found 14% higher 5-HT1A receptor binding (p = 0.045) and 21% lower SERT binding in BA 9 of individuals who had an MDE (p = 0.045).
Limitations: The study was limited by the small number of postmortem brain samples including BA 41/42 available for binding assays and the large overlap between suicide and depression in the MDE sample.
Conclusion: Depression may be associated with altered serotonergic function in the auditory cortex involving the 5-HT2A receptor and is part of a wider view of the pathophysiology of mood disorders extending beyond psychopathology.
Introduction
The temporal lobe, including the superior temporal gyrus, receives some of the highest-density serotonergic innervation in the telencephalon, with the primary auditory cortex receiving denser projections than the secondary auditory cortex.1,2 The serotonergic innervation occurs throughout all cortical layers.3 The loudness-dependent auditory evoked potential (LDAEP), an electroencephalography (EEG) measure originating from the primary auditory cortex, is linked to serotonergic tone in both humans and animals.4 Administration of selective serotonin reuptake inhibitors (SSRIs) and other compounds that increase serotonin, both systemically and locally in the auditory cortex, decrease the magnitude of the LDAEP.5–9 Taken together, these findings suggest that serotonin (5-HT) modulates auditory cortex function.
Serotonergic system abnormalities are implicated in a wide variety of mental health disorders, including major depressive disorder (MDD), bipolar disorder, anxiety disorders and schizophrenia. In MDD, the LDAEP correlates with responsiveness to antidepressant treatment.5,6,10 Depressed patients with larger LDAEPs at baseline have a greater response to SSRIs, and LDAEP magnitude correlates with the degree of improvement as measured by clinical ratings such as the Hamilton Rating Scale for Depression.6,11,12 These findings raise the possibility of functional differences in serotonergic function in the auditory cortex in MDD.
Beyond the LDAEP, our understanding of the role that 5-HT plays in the auditory cortex is limited. Animal studies have shown that 5-HT decreases GABAergic neurotransmission pre- and postsynaptically in the primary auditory cortex via 5-HT1A and 5-HT2A receptors, respectively, and decreases firing rate, input resistance and firing rate adaptation of pyramidal cells in juvenile rats.13,14 Although SSRI treatment has been associated with plasticity in the primary visual cortex,15 this association has not been observed in the primary auditory cortex.16 Tryptophan depletion studies in humans suggest that 5-HT affects early, attention-related processing of auditory stimulus information.7,17 Data on the effect of tryptophan depletion on LDAEP are mixed.7,18–20
The association between 5-HT, the neurophysiology of the auditory cortex and psychopathology remains to be understood. To better understand what effect 5-HT may be exerting in the auditory cortex and how this effect may be different in disease, we studied the expression of 5-HT1A receptors, 5-HT2A receptors and the serotonin transporter (SERT) in postmortem brain tissue sections containing auditory cortex of healthy nonpsychiatric controls and individuals who had a mood disorder and met criteria for a major depressive episode (MDE). Receptor binding was quantified in primary and secondary auditory cortical regions (Brodmann areas [BA] 41/42 and 22) compared with the primary motor cortex (BA 4) and prefrontal cortex (BA 9).
Methods
Details on the collection of brain tissue, toxicology, psychological autopsy and receptor autoradiography have been described in detail in previous publications21,22 and are only summarized here for convenience.
Subjects
Written informed consent was obtained from the next of kin of the deceased individuals from whom the samples were obtained. Both the nonpsychiatric controls and the individuals who had an MDE in the present study were chosen based on the availability of tissue samples with auditory cortex.
Collection of brain samples
Brains were collected in New York, NY, Pittsburgh, PA, and the Republic of Macedonia. The collection of brain samples and brain tissue use were approved by the respective institutional review boards. Brain sample collection procedures were standardized across all collection sites. Brain collection took place at the time of autopsy, within 24 hours of the time of death. Upon removal from the cranium, the dura mater was stripped, the brainstem was separated by a transverse cut just anterior to the superior colliculus and the cerebellum was removed. The brain was bisected and the right hemisphere was cut into slabs approximately 2 cm thick in the coronal plane and frozen in liquid Freon. Samples were then flash-frozen to −80°C and stored at that temperature until further processing. The left hemisphere was placed in formalin for neuropathological examination. A psychological autopsy was used to obtain DSM-IV Axis I and II diagnoses. Lifetime information on suicidal behaviour, medical illness, medications, family history and developmental history was obtained in the psychological autopsy.23 The inclusion criteria for controls were death by accident, homicide, or sudden natural causes; no psychiatric disorder as per DSM-IV criteria24 or history of suicide attempts; and negative toxicology for psychoactive drugs. The inclusion criteria for the MDE sample were Axis I diagnosis of MDD or bipolar disorder, with at least 1 lifetime MDE. Individuals who had an MDE and a history of alcohol use disorder (AUD) were included in this study.
Toxicology
Brain samples were screened for more than 30 prescription medications and drugs of abuse, and were quantified if present. Blood and urine were additionally screened for alcohol, antidepressants, barbiturates, benzodiazepines, cannabis, carbon monoxide, cocaine, opiates, methadone, amphetamines, phencyclidine and salicylates.
Brain sectioning and autoradiography
Brain blocks were sectioned at 20 μM thickness. Receptor autoradiography assays were performed on tissue sections as described previously.22,25,26 In this study, we considered sections that contained BA 4, BA 9, BA 41/42 and BA 22. Prior to incubation with radioligand, endogenous ligands were removed by incubation in buffers. Nonspecific binding was determined by examining adjacent sections that had been incubated with a selective displacer. Sections were washed in incubation buffer at 4°C, dipped in water, rapidly dried and transferred to a vacuum desiccator until ready for exposure to film. Sections were exposed for 4–12 weeks depending on the receptor. Dried slides were arranged in an x-ray film cassette with 3H-containing polymer standards (American Radiolabelled Chemicals). Serotonin transporter availability was quantified by incubation with 0.4 nM of [3H]Cyanoimipramine, using 10 μM sertraline to determine nonspecific binding.25 We measured 5-HT1A receptor availability by incubation with 2 nM [3H]8-OH-DPAT, using 1 μM of 5-HT to assess nonspecific binding.25 Finally, we measured 5-HT2A receptor availability by incubation with 2 nM [3H]Ketanserin, using 1 μM prazosin and 1 μM tetrabenazine to block α-1 and tetrabenazine sites. Nonspecific binding was determined using 1 μM mianserin.27
Autoradiograms were sampled using a computer-based image analysis system (MCID, Interfocus Imaging), as described previously.22 Regions were drawn using a pointing device. The sampled regions at this anatomic level included BA4, BA9, BA41/42 and BA22, as defined using the atlas of Byrd (unpublished), as previously described.22,25
Statistical analysis
All data analyses were completed using custom scripts written for MATLAB 2017R. Initially, the binding profiles in the gyri of each BA in the depressed and control samples were compared using a Student t test. To account for the fact that receptor levels can change with age and differ by sex, we also analyzed ligand binding data from each BA using a general linear model. In these models, ligand binding was designated as the dependent variable, and diagnosis, sex and age were independent variables. Binding data from the gyri and sulci of brain tissue samples were modelled separately. However, because findings between gyri and sulci were very similar, only gyrus binding data are presented here, unless otherwise noted. Because 5-HT1A receptor, 5-HT2A receptor and SERT binding has previously been shown to be influenced by alcoholism,28–31 we created separate general linear models using 5-HT1A, 5-HT2A and SERT binding as the dependent variables, and age, sex and AUD as independent variables for the MDE sample only. Similarly, because previously published work has suggested that serotonergic activity may differ between individuals with unipolar and bipolar depression,32 we used a general linear model to examine the effect of unipolar versus bipolar depression diagnosis, age and sex on 5-HT1A, 5-HT2A and SERT binding in the MDE sample only. There were no brain section samples from individuals who had bipolar depression who had binding data for all 3 receptor subtypes from BA 41/42; therefore, this latter general linear model could not be completed for this region.
Results
Demographic characteristics and other sample information
Brain tissue samples from 76 individuals were included in this study: 40 controls and 36 who had an MDE. The demographic characteristics of both groups are listed in Table 1. There were no significant differences between the groups with regard to age, sex, racial/ethnic background and exposure to childhood adversity. Women were underrepresented in both samples, as expected from a medical examiner collection of a population with many accident and suicide decedents. Most of the individuals in the MDE group (> 90%) died by suicide. This sample also included 4 people who met diagnostic criteria for bipolar disorder. Approximately one-third of the individuals with an MDE had a history of AUD. Appendix 1, available at jpn.ca/180190-a1, includes information on age, sex, race/ethnicity, diagnosis, cause of death, postmortem interval, brain pH and brain toxicology for individuals in each group.
Demographic and clinical characteristics of participants in the study
5-HT2A receptor binding
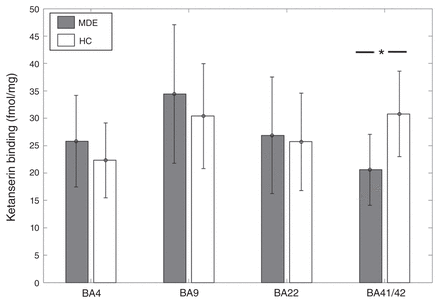
[3H]Ketanserin binding was 33% lower in BA41/42 of individuals who had an MDE (n = 8) than in controls (n = 12) (MDE mean 20.59 ± 6.5 fmol/mg tissue; controls mean 30.78 ± 7.8 fmol/mg tissue; t = −3.05, p = 0.0069; Fig. 1). In the general linear model, when accounting for the effects of age and sex, this difference remained significant with an inverse association between [3H]Ketanserin binding and MDE (t = −2.44, p = 0.027). [3H]Ketanserin binding did not differ significantly between the MDE and control samples in BA 22 (27 MDE, 31 controls, t = 0.23, p = 0.8), BA 4 (24 MDE, 23 controls, t = 1.56, p = 0.13) and BA 9 (28 MDE, 35 controls, t = 1.44, p = 0.15). However, in the general linear model, accounting for age and sex, BA 4 showed a trend-level positive association between [3H]Ketanserin binding and MDE (t = 1.71, p = 0.095).
[3H]Ketanserin binding across Brodmann area (BA) 4, BA 9, BA 22, and BA 41/42 in healthy controls (HC) and individuals with a history of major depressive episodes (MDE). [3H]Ketanserin binding was 33% lower in BA41/42 of the MDE compared with the control group (MDE mean 20.59 ± 6.5 fmol/mg, HC mean 30.78 ± 7.8 fmol/mg; t = −3.05, p = 0.0069). There were no significant differences in BA 4 (MDE mean 24.81 ± 8.35 fmol/mg, HC mean 22.32 ± 6.84 fmol/mg), BA 9 (MDE mean 34.44 ± 12.64 fmol/mg, HC mean 30.4 ± 9.57 fmol/mg), and BA 22 (MDE mean 25.86 ± 10.65 fmol/mg, HC mean 24.76 ± 8.75 fmol/mg).
Within the MDE sample, [3H]Ketanserin binding showed a positive association with AUD in BA 41 (t = 3.65, p = 0.022) and BA 22 (t = 2.3, p = 0.025). To account for the effect of AUD in the comparison between the MDE and control samples, AUD was included as an additional independent variable in the general linear model. This did not significantly change the negative association between MDE and [3H]Ketanserin binding (t = −2.86, p = 0.012). [3H]Ketanserin binding did not show any association with bipolar depression in any BA other than a trend-level positive association in BA 9 (t = 1.84, p = 0.078).
The general linear model for BA 22 showed a significant positive association between male sex and [3H]Ketanserin binding (t = 3.3, p = 0.0017), indicating higher binding in males, as well as an inverse association with age (t = −4.15, p = 0.00011). In BA 9 and BA 4, the general linear model showed an inverse association between age and [3H]Ketanserin binding (BA 9: t = −3.93, p = 0.00022; BA 4: t = −3.31, p = 0.0019).
5-HT1A receptor binding
In BA 9, [3H]8-OH-DPAT binding was 14% higher in the MDE sample (n = 29) than the control sample (n = 31) (MDE mean 16.07 ± 3.92 fmol/mg; control mean 13.98 ± 3.99 fmol/mg, t = 2.05, p = 0.045; Fig. 2). However, after accounting for sex and age with the general linear model, this result became a trend-level finding in gyrus data (t = 1.79, p = 0.079). There was no significant difference in [3H]8-OH-DPAT binding in BA 41 (6 MDE, 2 controls, t = 1.28, p = 0.25), BA 22 (33 MDE, 34 controls, t = 1.46, p = 0.15), or BA 4 (30 MDD, 26 controls, t = 1.23, p = 0.22; Fig. 2).
[3H]8-OH-DPAT binding across Brodmann area (BA) 4, BA 9, BA 22, and BA 41/42 in healthy controls (HC) and individuals with a history of major depressive episodes (MDE). [3H]8-OH-DPAT binding was 14% higher in BA 9 of the MDE group compared with the control group (MDE mean 16.07 ± 3.92 fmol/mg, HC mean 13.98 + 3.99 fmol/mg, t = 2.05, p = 0.045). However, after accounting for the effects of age and sex, this finding became a trend-level observation (t = 1.79, p = 0.079). There was no significant difference between the MDE group and the control group in BA 4 (MDE mean 12.17 ± 5.45 fmol/mg, HC mean 10.56 ± 4.15 fmol/mg), BA 22 (MDE mean 16.13 ± 6.44 fmol/mg, HC 14.07 ± 5.38 fmol/mg), and BA 41/42 (MDE mean 12.84 ± 3.42 fmol/mg, HC mean 9.47 ± 1.94 fmol/mg).
The generalized linear model for [3H]8-OH-DPAT binding in BA 22 showed a significant inverse association with age in gyrus binding measures (t = −2.93, p = 0.0047), but only a trend-level association was found with sulcus binding measures (t = −1.87, p = 0.066). In BA 9 there was an inverse association between [3H]8-OH-DPAT binding and age (t = −2.18, p = 0.033), and in sulcus binding measures there was an inverse association with male sex, such that males had lower binding (t = −2.14, p = 0.036). In BA 4, there was a positive association between [3H]8-OH-DPAT binding and male sex in sulcus data (t = 2.25, p = 0.029), and there were trend-level associations for age in gyrus and sulcus (t = −1.99, p = 0.051). [3H]8-OH-DPAT binding did not show any association with AUD or bipolar depression in BA 22, BA 9 and BA 4. The data were insufficient to assess the effects of AUD and bipolar depression in BA 41.
SERT binding
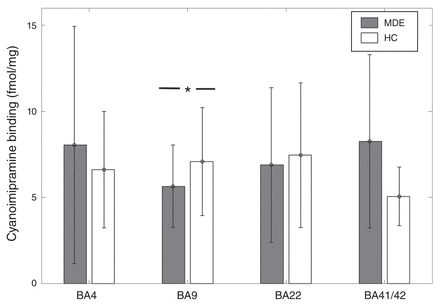
[3H]Cyanoimipramine binding was 21% lower in BA 9 in the MDE sample (n = 30) than in the control sample (n = 35) in sulcus data (MDE mean 5.65 ± 2.4 fmol/mg; controls mean 7.09 ± 3.14 fmol/mg, t = −2.04, p = 0.045; Fig. 3). This finding remained significant after accounting for the effects of age and sex with the general linear model (t = 2.13, p = 0.037). [3H]Cyanoimipramine binding did not differ significantly between the MDE and control samples in BA 41/42 (7 MDE, 6 controls, t = 0.78, p = 0.45), BA 22 (26 MDE, 26 controls, t = −0.89, p = 0.37) and BA 4 (22 MDE, 23 controls, t = 0.61, p = 0.53; Fig. 3).
[3H]Cyanoimipramine binding across Brodmann area (BA) 4, BA 9, BA 22, and BA 41/42 in healthy controls (HC) and individuals with a history of major depressive episodes (MDE). [3H]Cyanoimipramine binding was 21% lower in BA 9 in the MDE group compared with the control group in sulcus data (MDE mean 5.65 ± 2.4 fmol/mg, HC mean 7.09 ± 3.14 fmol/mg, t = −2.04, p = 0.045). There was no significant difference between the MDE group and the control group in BA 4 (MDE mean 8.04 ± 6.93 fmol/mg, HC mean 6.61 ± 3.4 fmol/mg), BA 22 (MDE mean 6.89 ± 4.53 fmol/mg, HC mean 7.45 ± 4.25 fmol/mg), and BA 41/42 (MDE mean 8.26 ± 5.04 fmol/mg, HC mean 5.05 ± 1.69 fmol/mg).
[3H]Cyanoimipramine binding showed a negative association with male sex in gyrus data in BA 22 (t = −2.53, p = 0.015) and BA 41/42 (t = −2.26, p = 0.05). Binding also showed a negative association with age in BA 22 gyrus data (t = −2.3, p = 0.026) and both gyrus and sulcus data in BA 9 (t = −2.18, p = 0.033). There was a trend-level positive association between AUD and [3H]Cyanoimipramine binding in BA 9 (t = 1.75, p = 0.092) and BA 4 (t = 1.86, p = 0.079). There was no association between [3H]Cyanoimipramine binding and bipolar depression in any BA.
Discussion
In this study, we found that individuals who had a history of MDE had lower 5-HT2A receptor binding, specifically in BA 41/42, where the primary auditory cortex is located. Although we did not perform measurements to determine whether these changes were due to lower numbers of receptors or changes in affinity of receptors, previous studies using brain homogenates support the former conclusion.33–35 This observation indicates perturbations in serotonergic function in MDD in cortical, early-stage auditory processing areas. This finding is consistent with previous data that indicate that primary auditory cortex responses are modulated by 5-HT availability5,7–9 and that these cortical responses may predict treatment response in MDD.6,10,11 Furthermore, a similar finding in postmortem tissue of patients with schizophrenia has been reported.36
While modulation of auditory responses by 5-HT has been observed as early in the auditory processing pathway as the inferior colliculus,37–40 our finding also suggests that modulation of auditory cortical responses is not merely carried forward from lower processing regions. To date, the receptors and specific cell types that underlie the serotonergic modulation of the loudness-dependent response in the primary auditory cortex remain unclear, though previous work has implicated both 5-HT1A receptors41 and SERT.42 Our work suggests that the role of 5-HT2A receptors should also be examined.
We did not observe significant differences between controls and depressed individuals in 5-HT2A receptor binding in other control regions examined, most notably in the prefrontal cortex (BA 9), which is consistent with the results of some previous studies.43–47 However, other studies have reported increased 5-HT2A receptor levels in the prefrontal cortex of individuals who died by suicide.33–35,48–50 The 5-HT2A receptor binding in these regions may be modulated by aggression and whether the individual died by violent suicide.45,47 As in other studies, the effect of depression is difficult to separate from the effect of suicide in our sample, given that most of the individuals in our MDE sample died by suicide.
In addition, we observed lower levels of SERT binding and higher levels of 5-HT1A receptor binding in BA 9 in the MDE sample. However, the latter finding did not remain significant after accounting for the effect of age and sex. Lower SERT binding in BA 9 is consistent with the findings of previous postmortem studies, which reported lower prefrontal cortex SERT binding in depression and suicide.25,31,51 Lower SERT binding has also been described previously in midbrain,52–55 anterior cingulate and subcortical regions55,56 in positron emission tomography studies of individuals with MDD. However, others have also reported no differences or higher binding in these regions.57–60 Similarly, our finding of higher 5-HT1A receptor binding in BA 9 is consistent with those of some previous postmortem61 and positron emission tomography studies,62–66 though others have differed in their findings.67–73 Overall, these findings may indicate adaptations to changes in serotonergic tone in the prefrontal cortex, suggesting decreased 5-HT availability.
Adaptive changes in depression may be regionally specific, given that lower 5-HT2A receptor binding was observed only in the primary auditory cortex, but not in other regions. This may relate to the specific functional role that these receptors play in various cortical regions. Although the specific role of 5-HT2A receptors in the auditory cortex has yet to be clarified, it has been suggested that they play an important role in postnatal cortical development.74 In mature auditory cortex, 5-HT2A receptors contribute tracking of dynamic tonal structure in sound.75 Several hallucinogens, such as lysergic acid diethylamide (LSD), are 5-HT2A receptor agonists and are known to produce auditory hallucinations and perturbations. It is notable that lower 5-HT2A receptor levels have also been found in the auditory cortex of patients with schizophrenia,36 a disease characterized by hallucinations and in which serotonergic dysfunction is also thought to play an important role given the serotonergic activity of many second-generation antipsychotic medications.
Limitations
Limitations of this study include the small number of postmortem brain samples including BA 41/42 available for binding assays. Despite this limitation, our analysis yielded a significant finding. However, the study was not adequately powered to correct for multiple comparisons, which we did not perform in these analyses. In addition, the effect of depression was confounded by the effect of suicide and aggression, as indicated by violent suicide deaths, in this sample. Therefore, this study should be considered preliminary; further replications of this finding are needed.
Conclusion
We present here a preliminary but novel finding of lower 5-HT2A receptor binding in the primary auditory cortex of individuals in an MDE at the time of death. This finding supports previous functional data that indicate that depression is associated with changes in the primary auditory cortex. Furthermore, our results suggest that adaptive changes in depression may be regionally specific, given that lower 5-HT2A receptor binding was observed only in the primary auditory cortex, whereas changes in SERT binding were confined to BA 9 in this study. Our data support further study of potential biomarkers of depression in auditory processing regions.
Acknowledgements
The authors thank Dr. Steve Ellis for his feedback on the statistical analysis of the data.
Footnotes
Competing interests: J. Mann receives royalties from the Research Foundation of Mental Hygiene for commercial use of the C-SSRS. No other competing interests declared.
Contributors: L. Steinberg, M. Underwood, J. Mann and V. Arango designed the study. M. Underwood, M. Bakalian, S. Kassir, J. Mann and V. Arango acquired the data, which L. Steinberg, M. Underwood, M. Bakalian, J. Mann and V. Arango analyzed. L. Steinberg and M. Bakalian wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
- Received October 15, 2018.
- Revision received February 4, 2019.
- Accepted February 17, 2019.