Abstract
Background: There is increasing evidence that people with attention-deficit/hyperactivity disorder (ADHD) are impaired in emotion regulation, but psychophysiological and functional MRI data on emotion processing in adult patients with ADHD are scarce. We investigated the neural correlates of reappraisal as one of the most efficient emotion-regulation strategies.
Methods: We included 30 adult patients with ADHD and 35 healthy controls in our study. We applied a well-established reappraisal paradigm in functional MRI and assessed behavioural emotion-regulation strategies with standardized questionnaires. We hypothesized that patients with ADHD would demonstrate impaired reappraisal related to reduced activations in the frontoparietal cognitive control network.
Results: Despite our hypothesis, we found no significant activation differences in the neural reappraisal network between patients with ADHD and controls. As well, both groups revealed similar reappraisal success on the immediate behavioural ratings in the scanner. Interestingly, patients with ADHD revealed significantly increased activations in the dorsal and ventral anterior cingulate cortex (ACC) compared with controls when viewing negative > neutral pictures. These ACC activations were significantly correlated with the prevalence of habitual use of reappraisal in patients with ADHD only.
Limitations: Patients withdrew medication only 24 hours before the experiment; we investigated negative, but not positive, emotion processing and regulation.
Conclusion: Although emotion dysregulation is regarded as a core symptom of ADHD, explicit reappraisal does not seem to be impaired in adult patients. However, increased activation of the ACC implies stronger implicit emotion regulation induced by negative stimuli. This might be explained by emotional hyperresponsivity in patients with ADHD compared with controls.
Introduction
Attention deficit/hyperactivity disorder (ADHD) is the most prevalent neurodevelopmental psychiatric disorder in childhood, and about one-third of patients have persistent symptoms into adulthood.1 Large epidemiological studies have yielded a prevalence of approximately 2.5% in adults.2,3 The severity of the disorder in adulthood has been shown by a large number of studies revealing problems in social and professional life (e.g., a greater number of unstable partnerships, significantly lower levels of education and higher university dropout rates).4,5
A core feature of ADHD is emotional dysregulation.6–9 Emotion regulation consists of the modulation of physiologic and behavioural correlates of an emotional response after a positive or negative emotional stimulus.10 In a comprehensive review of studies focusing on self-report data about emotion-regulation difficulties in adults and children with ADHD, Shaw and colleagues8 reported prevalence rates for emotional dysregulation of 34% to 70% in adults with ADHD. Furthermore, significantly higher rates of emotional dysregulation were found in people with ADHD compared with healthy participants,7 especially with respect to anger.11 Crucially, ADHD with emotional dysregulation has been associated with more severe ADHD presentations,12 higher persistence and comorbidity rates,13 and worse social and occupational adjustment7 compared to ADHD without emotional dysregulation.
Theoretical frameworks posit 2 main areas of emotional dysfunction in patients with ADHD: heightened emotional reactivity and deficient top–down regulation of emotion.9,14,15 Consistent with the concept of altered emotional reactivity, previous functional MRI (fMRI) studies reported enhanced processing of emotional distractors during executive tasks in ADHD, reflected in valence-specific differential neural activation patterns in emotion regulation and salience brain networks.16,17 Posner and colleagues reported increased activations of the medial prefrontal cortex (PFC),18 whereas Passarotti and colleagues observed increased activations of the dorsolateral PFC and parietal cortex and decreased activations of the ventrolateral PFC during an emotional Stroop task in patients with ADHD.19 The processing of fearful and neutral facial stimuli has been associated with increased amygdala activation.20,21 It is noteworthy, however, that most studies on emotion regulation and processing have been conducted in pediatric or adolescent patients, which makes inference to adults difficult, given the developmental changes pertinent to the disorder and the ability to regulate emotions.1,17,22 Moreover, while there is some evidence of altered emotional reactivity and interference control in ADHD, to our knowledge no MRI study has investigated the neural correlates of emotional dysregulation using a paradigm that directly targets emotion regulation. In our recent electrophysiological study on emotion regulation in patients with ADHD, we found elevated frontal late positive potential amplitudes during the viewing of negative pictures and during emotion regulation using reappraisal.23
Explicit emotion-regulation strategies include reappraisal, distraction and suppression. Reappraisal is the intentional reinterpretation of an emotion-eliciting stimulus to down- or upregulate the related emotional response.24 It is the most well-studied emotion-regulation strategy and has repeatedly been associated with robust activations in cognitive control regions, including the ventrolateral and dorsolateral PFC, dorsal anterior cingulate cortex (ACC), inferior/superior parietal cortex, supplementary motor area, insula and striatum.25,26 Furthermore, habitual reappraisal compared with other emotion-regulation strategies has been associated with longer-lasting effects on emotions27–29 and better interpersonal functioning and well-being.30,31 Consequently, impaired reappraisal abilities in patients with ADHD present a promising target for treatment.
To shed light on the neural correlates of reappraisal in ADHD, we conducted an fMRI study using a reappraisal task adapted from Wager and colleagues.32 Based on the literature, we expected patients with ADHD to exhibit altered activations in the reappraisal network related to less successful downregulation of emotional responses.
Methods
Participants
We recruited 32 adult patients with ADHD from the outpatient clinic of the Departments of Psychiatry and Psychology at the University of Münster and 35 age- and sex-matched healthy controls using advertisements in local newspapers and Internet announcements. The sample partially overlapped with participants in our previous study using event-related potential techniques to investigate emotion regulation in ADHD.23
Exclusion criteria for both groups were as follows: psychotic disorder, bipolar disorder, severe major depressive episode within the last 5 years, obsessive–compulsive disorder, substance use disorder, neurologic disorders, serious head injury and IQ < 80. For healthy controls, exclusion criteria were current or past psychiatric disorders and above-threshold symptoms of ADHD. One patient was excluded because of current major depressive disorder, and another patient terminated MRI scanning prematurely. The final sample consisted of 30 patients with ADHD (20 combined subtype and 10 inattentive subtype) and 35 healthy controls.
All participants were screened using the Adult ADHD Self-Report Scale (ASRS).33 In patients with ADHD, diagnoses were verified by a trained clinical psychologist or psychiatrist with the Diagnostic Interview for ADHD in Adults (DIVA 2.0).34 We assessed current ADHD symptom severity and childhood manifestation of ADHD using the German version of the ADHD Self-Report Scale (ADHD-SB)35 and the German short version of the Wender Utah Rating Scale (WURS-K),36 respectively. We evaluated all participants for psychiatric (co)morbidity using the Structured Clinical Interview for DSM-IV (SCID).37 Comorbid disorders in the ADHD group included dysthymic disorder (n = 2), panic disorder (n = 1) and social phobia (n = 1).
We estimated intellectual ability using the Vocabulary and Matrix Reasoning subtests from the German version of the Wechsler Adult Intelligence Scale (WAIS-IV)38 and assessed attention using the Frankfurt Attention Inventory (FAIR-2).39 We analyzed emotion-regulation strategies using the German version of the Emotional Regulation Questionnaire (ERQ).40 For participants’ self-appraisal of emotional competence, we used a German self-report questionnaire (SEK-27).41 We assessed depressive symptoms using the Beck Depression Inventory II (BDI-II).42
Nine patients with ADHD were taking stimulant medications (methylphenidate or amphetamine sulfate). These patients discontinued their stimulant medication 24 hours before scanning. The study was carried out in accordance with the latest version of the Declaration of Helsinki (2013) and was approved by the local medical ethics committee (Ethics Committee of the Medical Council of Westphalia and the Westphalia Wilhelms-University Muenster). All participants provided written informed consent and were financially reimbursed for their participation in the study at an hourly rate of €10.
Stimuli and procedure
For the fMRIs, we adapted a reappraisal task developed by Wager and colleagues.32 We selected pictures from the International Affective Picture System43: 16 negative images ( valence [mean ± standard deviation (SD)] 2.36 ± 0.69, arousal 5.89 ± 0.48; codes 1113, 1070, 2703, 2800, 6415, 9500, 6570, 6563, 3181, 3180, 9075, 9040, 9520, 9530, 9920 and 9908) and 8 neutral images (valence 5.04 ± 0.20, arousal 2.97 ± 0.49; codes 2214, 2383, 2026, 2495, 2850, 7035, 7080 and 7178). Participants were asked to view (neutral and negative pictures) or reappraise (negative pictures only). Negative pictures were counterbalanced across the negative view and reappraise conditions. Two versions of the pictures were randomly applied: negative images from the negative view and reappraise conditions in version A were exchanged with their corresponding images in version B. The 2 sets were matched for valence (t = −0.17, p = 0.87) and arousal (t = 0.33, p = 0.74). Prior to scanning, participants received detailed instructions for the emotion-regulation task. For the reappraise task, participants were asked to decrease their negative emotional response by reinterpreting the depicted event in a less negative manner (e.g., a person is trying to help or comfort an injured person). Each participant completed several training trials to correctly understand the reappraisal technique.
Stimuli were presented with an fMRI projection system using Inquisit 3 software.44 Each trial started with the instruction “view” or “reappraise” presented for 2000 ms, followed by a fixation cross displayed for 4000 ms. Neutral or negative pictures were shown for 10 000 ms. For the view condition, the instruction was presented on a light grey background and the image was framed in the same colour. For the reappraise condition, the instruction background and image frame were light green. After each view or reappraise condition, a back screen was displayed, jittered between 2000 and 5000 ms, followed by a written instruction (“How do you feel?”) for 10 000 ms; during this period, participants rated the degree to which they had experienced a negative emotional reaction to the preceding picture on a 4-point Likert scale (neutral, slightly negative, very negative, and extremely negative). Ratings were performed by pressing 1 of 4 buttons (2 on the right side and 2 on the left side) connected to a response box using optical fibres. Finally, a fixation cross with a jittered duration of 4000 ms to 7000 ms was presented before the next trial was initiated. The sequence of trials was randomly arranged. Participants completed 8 trials in each condition, for a total of 24 trials. The duration of the experiment was 11 minutes, 36 seconds.
Image acquisition
We acquired whole-brain blood oxygen level–dependent fMRI data using a 3 T scanner (PRISMA Fit, Siemens). For functional T2*-weighted images, we used an echo-planar single-shot gradient echo pulse sequence (matrix 64 mm × 64 mm, repetition time 3000 ms, echo time 30 ms, field of view 240 mm, 30 axial slices at 3.5 mm). In the same session, we acquired a high-resolution T1-weighted anatomic image to aid with spatial normalization (3D MP-RAGE; matrix 256 × 256, repetition time 2130 ms, echo time 2.3 ms, inversion time 900 ms, flip angle 8°, field of view 256 mm, 196 sagittal slices at 1.0 mm).
Functional MRI analyses
Preprocessing
We analyzed the anatomic T1 and functional T2* images using SPM12 (Wellcome Department of Cognitive Neurology). First, functional images were realigned to correct for rigid body motion. Then, the individual anatomic image was coregistered to the mean functional image computed during realignment. High-resolution anatomic images were normalized to Montreal Neurological Institute (MNI) space using the unified segmentation algorithm. The normalization parameters from the previous step were applied to the functional images, which were then resliced to a resolution of 2 mm3. Finally, functional images were smoothed using an isotropic 8 mm3 full-width at half-maximum Gaussian kernel. The individual mean functional images were segmented, and a brain mask for the whole group was computed using grey and white matter segments.
Data analyses
On the first level, individual general linear models were specified using regressors for “neutral view,” “negative view,” “reappraise,” “instructions,” “ratings” and the 6 movement parameters from the realignment. The models were estimated for the voxels specified by the explicit brain mask that was computed during preprocessing. Activation maps for the 3 conditions of interest were computed using simple t contrasts. On the second level, we specified a mixed analysis of variance (ANOVA) model with the between-subject factor group (ADHD v. controls) and the within-subject factor condition (neutral view, negative view or reappraise), and we used subsequent t contrasts. We based the whole-brain analysis of main effects on family-wise error (FWE)–corrected voxels at pFWE < 0.05 and used a cluster-wise (CWC) FWE correction for multiple comparisons at pCWC,FWE < 0.05 and a cluster extent threshold of k = 20. The main effects were evaluated using the contrasts negative view > neutral view and reappraise > negative view. The whole-brain analysis of the interaction effects was done using an uncorrected p = 0.001 and a cluster extent of 240 voxels to assure a corrected FWE rate for clusters of p < 0.05 as recommended by Eklund and colleagues45 and Woo and colleagues.46 We evaluated the interaction effects comparing the aforementioned contrasts between groups. Significant clusters from statistical parametric maps were anatomically labelled by examining the MNI coordinates using the AAL toolbox for SPM12.47,48
To test the robustness of our results, we recalculated the ANOVA and contrasts described above using potential nuisance variables as covariates. To control for the effects of methylphenidate, we included medication (yes/no) as a covariate. To control for the effects of depression, we included the BDI-II sum score as a covariate.
Correlations
A repeated criticism is that brain–behaviour correlations have shown inflated and unrealistically high correlation coefficients.49,50 To avoid inflation because of multiple testing, we restricted our analyses to clusters that were significantly different between patients and controls for the 2 main effects (negative view > neutral view and reappraise > negative view). From the second-level model, we extracted the first eigenvariate of the respective cluster of the con-image for each participant and contrast. Eigenvariate values were correlated with the subscales of the ERQ (suppression and reappraisal) using Pearson coefficients. We calculated the correlation coefficients for both groups separately, tested each of them against the null hypothesis and compared the group coefficients using Fisher z.
Behavioural data
To compare groups regarding the clinical data, we used independent sample t tests. We carried out the analyses with SPSS version 24.0.0.1 (SPSS Inc.). We set p < 0.05 as the threshold for statistical significance. We analyzed the ratings of the images in the scanner using mixed-model repeated-measures ANOVA using group (ADHD and healthy controls) and condition (neutral view, negative view and reappraise). Owing to a technical error of the response box during testing, the ratings of 3 healthy controls were not included in the analyses. Significant main effects were followed up using post hoc t tests with Bonferroni-adjusted p values.
Results
Participants
Demographic and clinical sample characteristics are presented in Table 1. Patients did not differ with respect to any demographic variable except for a slightly reduced mean years of education in the ADHD group compared with healthy controls.
Study sample characteristics
Functional MRI
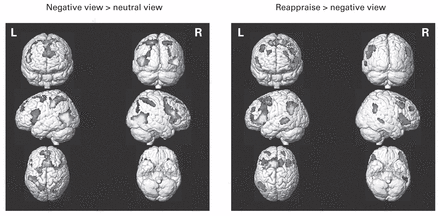
First, we evaluated the main effects of viewing negative images compared with neutral images and reappraising negative images compared with viewing negative images as a proof of the validity of the paradigm. The contrast negative view > neutral view revealed significant activations in a widely distributed neural network encompassing 4 large clusters in the frontal, temporal, parietal and occipital lobes (left side of Fig. 1 and Appendix 1, Table S1, available at jpn.ca/180139-a1). More precisely, activations were located bilaterally in the superior and inferior frontal gyri, the superior and inferior parietal gyri, the middle occipital gyri and the middle temporal gyri, as well as in the left insula, left precuneus, left supplementary motor area and left postcentral gyrus. The contrast reappraise > negative view revealed significant activations in a neuronal network made up of the frontotemporoparietal lobes (right side of Fig. 1 and Appendix 1, Table S2). The clusters included the bilateral superior, middle and inferior frontal gyri; the insula; the bilateral middle temporal gyri and the right superior temporal gyrus; the inferior parietal gyri; the angular gyri; the supplementary motor area; the right supramarginal gyrus; and the left caudate nucleus and putamen.
Main effects of negative view and reappraisal. The displayed clusters survived correction for multiple comparisons at pCWC,FWE < 0.05, at a cluster extent threshold of k = 20 based on corrected voxels at pFWE < 0.05. The contrast maps are projected onto the normalized MRIcron template brain. CWC = cluster-wise correction; FWE = family-wise error correction.
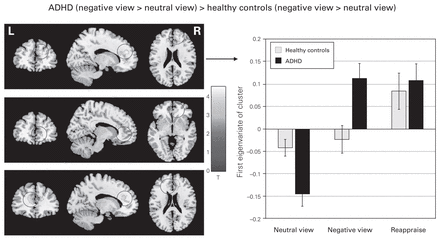
Next, we analyzed the main effects of group and the interaction effects of group × condition. We found no significant main effects of group; neither the contrast ADHD > healthy controls nor the opposite comparison revealed any significant activation. Only 1 interaction effect reached significance: the comparison ADHD (negative view > neutral view) > healthy controls (negative view > neutral view) resulted in 3 clusters in the dorsal and ventral ACC (Fig. 2 and Table 2). This interaction effect proved to be robust. The analysis of covariance (ANCOVA) encompassing the covariate medication essentially reproduced the results. The first cluster in the right ACC was almost identical (pFWE = 0.009, k = 387). However, clusters 2 and 3 from the ANOVA emerged as 1 large cluster in the ANCOVA (pFWE = 0.001, k = 619). The ANCOVA encompassing the covariate depression revealed 1 large significant cluster in the left and right ACC (pFWE < 0.001, k = 1118) encompassing the 3 clusters from the ANOVA. In summary, neither medication nor depression was responsible for the depicted interaction effect.
Interaction effect for patients with ADHD (negative view > neutral view) > healthy controls (negative view > neutral view). The reported clusters survived correction for multiple comparisons at pCWC,FWE < 0.05, at a cluster extent threshold of k = 240 based on uncorrected voxels at p < 0.001. The contrast maps are projected onto the normalized MRIcron template brain. The bar graph depicts the eigenvariates of the first cluster in the right dorsal ACC. The bars represent activation of the cluster during all 3 conditions, but the contrast was significant only for the interaction. This figure shows that this area of the ACC was similarly activated in both groups during reappraisal. ACC = anterior cingulate cortex; ADHD = attention-deficit/hyperactivity disorder.
Interaction effect, patients with ADHD (negative view > neutral view) > healthy controls (negative view > neutral view)
Because 4 of the patients with ADHD had comorbidities, we reanalyzed our data and excluded these 4 patients; we found no differences in our results.
Analyses of ratings in the scanner after each trial revealed a significant main effect of condition (F2,59 = 65.37, p < 0.001, ηp2 = 0.69). Post hoc tests showed that negative images induced a significantly more negative emotion (mean ± SD, 2.92 ± 0.68) than neutral images (3.89 ± 0.20; t61 = 11.58, p < 0.001, d = 1.804), and negative reactions to negative pictures were significantly less negative in the “reappraise” condition (3.30 ± 0.55) than in the “negative view” condition (t64 = 6.01, p < 0.001, d = 0.55), indicating successful reappraisal. The effects of group and the group × condition interaction were not significant.
To better delineate the power of our study, we performed a computation of the required sample size, which may help to assess the validity of our data. The required minimum sample size (n ≥ 16) to obtain a power of at least 0.95 was estimated using G*Power (f = 0.5, α = 0.05, β − 1 > 0.95, 2 groups, 2 conditions, r = 0.5).51 We inferred the effect size from the meta-analyses of Picó-Pérez and colleagues52 and Wang and colleagues.53
Questionnaires
Healthy controls reported significantly higher use of reappraisal strategies (ERQ reappraisal: ADHD 22.97 ± 7.58, healthy controls 27.14 ± 6.05, t = 2.40, p = 0.020) while ADHD patients engaged in suppression more regularly (ERQ suppression: ADHD 15.38 ± 5.37, healthy controls 11.40 ± 4.10, t = −3.28, p = 0.002). As well, patients with ADHD showed elevated depression scores compared with healthy controls (BDI-II: ADHD 16.00 ± 9.94, healthy controls 2.40 ± 2.90, t = −7.72, p < 0.001) and estimated their emotional competence more negatively than healthy controls (SEK-27: ADHD 54.29 ± 13.98, healthy controls 81.70 ± 12.29, t = −8.06, p < 0.001).
Correlations
We did not find significant activation differences between patients and controls for reappraise > negative view, so we did not perform any further analyses.
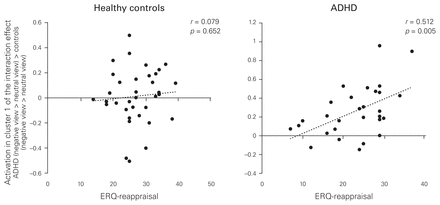
As reported before, in the contrast negative view > neutral view, 3 clusters in the ACC were significantly more strongly activated in patients with ADHD than in healthy controls. Therefore, we correlated these significant activations with the subscales of the ERQ in ADHD patients and controls separately; the habitual preference for reappraisal correlated positively with all 3 clusters in patients with ADHD but not in healthy controls, and 2 of the coefficients were significantly larger in patients with ADHD than in healthy controls (Table 3). As an example, the correlations of habitual reappraisal with the first cluster in the ACC are shown in Figure 3. Habitual suppression did not correlate with the activated clusters, in healthy controls or in patients with ADHD.
Positive correlations between self-reported habitual reappraisal (Emotion Regulation Questionnaire) and activation in the dorsal anterior cingulate cortex for ADHD (negative view > neutral view) > healthy controls (negative view > neutral view) in patients with ADHD and healthy controls, separately. The activation was extracted using the first eigenvariate of the first (largest) cluster. ADHD = attention-deficit/hyperactivity disorder; ERQ = Emotion Regulation Questionnaire.
Correlation of habitual reappraisal (Emotion Regulation Questionnaire) with the 3 activated clusters of the interaction effect, patients with ADHD (negative view > neutral view) > healthy controls (negative view > neutral view)*
Discussion
This study was the first to investigate the neural correlates of emotion regulation related to reappraisal in adults with ADHD compared with healthy controls using fMRI. Contrary to our hypothesis, we did not find significant differences in the neural reappraisal networks between patients with ADHD and healthy controls. Additionally, at a behavioural level, reappraisal equally attenuated the negativity ratings of emotional pictures in both groups. Thus, our data do not support the hypothesis of impaired emotion regulation in patients with ADHD when explicit strategies such as reappraisal are used. Overall, the activation patterns related to reappraisal in both groups closely comprised the reappraisal network described by Buhle and colleagues,26 Kohn and colleagues54 and Ochsner and colleagues.25 In these meta-analyses, reappraisal strategies were consistently related to cognitive control regions, including the dorso- and ventrolateral PFC, the medial PFC including the dorsal ACC, the posterior parietal gyri, the angular gyrus and the temporal gyri, brain regions that are in line with psychological models emphasizing the role of domain-general cognitive control processes in the regulation of emotion.55
Interestingly, patients with ADHD showed increased activations in the dorsal and ventral ACC compared with healthy controls during the presentation of negative images contrasted with neutral images. In this view condition, participants were asked to focus on the images and not avoid looking at them. In this way, they were asked to prevent the use of an explicit emotion-regulation strategy: distraction or attention deployment. Therefore, the observed neural activation differences in the dorsal and ventral ACC relate to differential emotional reactivity and/or processing of negative stimuli in patients with ADHD and healthy controls.
Subdivisions of the ACC have been implicated in different emotional processes. While the dorsal ACC is associated with the (re)appraisal of emotional stimuli and emotion generation, the ventral ACC (further divided into the pregenual and sub-genual ACC) is associated with the inhibition and suppression of emotions.56,57 Additionally, the computational model of emotion regulation by Etkin and colleagues58 places the ventral ACC–ventromedial PFC in the centre of (model-free) implicit emotion regulation characterized by decisions based on prediction error: an efficient, fast, but not very flexible strategy. Consequently, activation of the ventral ACC–ventromedial PFC reflects experience-dependent changes in the value of emotion-regulation behaviour based on prediction error feedback. Model-based control is characterized by application of rule-based decision making based on the individual’s a priori knowledge of the context (e.g., reappraisal). Model-based control is less computationally efficient and is applied when adjustments based on prediction error do not accomplish the same result. Thus, increased activation in the ventral ACC in patients with ADHD versus healthy controls relates to enhanced implicit regulation of emotional responses elicited by negative images, most likely related to an emotional hyperresponsitivity in patients with ADHD. The idea of emotional hyperresponsitivity in ADHD is supported by previous research in adolescents and children.18,21
Recently, Braunstein and colleagues59 extended their model with respect to the nature of the regulation goal and the nature of the emotion change process. They defined 2 orthogonal dimensions (explicit versus implicit and automatic versus controlled) as a framework for all emotion-regulation processes. Whereas reappraisal would be an explicit and controlled emotion-regulation strategy, emotional reactions would relate dimensionally to more incidental (implicit) goals with more automatic change processes. In our study, where no explicit emotion-regulation goal had to be achieved in the negative view condition, activation in the ventral ACC–ventromedial PFC would again relate to implicit emotion regulation, whereas activity in the dorsal ACC would account for the use of cognitive control mechanisms in implicit or explicit emotion regulation.
Another explanation for increased dorsal ACC activity while attending to negative stimuli might be that patients had more difficulty following the alternating instructions of “view” and “reappraise.” Despite the fact that we used different colour coding for the 2 instructions to support a prompt reaction, impulsivity and impaired inhibition in patients with ADHD might have fortified reappraisal strategy expectations as the more demanding task. Nevertheless, this seems unlikely because on a behavioural level patients with ADHD and healthy controls did not differ in their negativity ratings of induced emotions by negative pictures.
In our study, we did not find increased amygdala activation in patients with ADHD, as would be expected for emotional hyperresponsitivity (e.g., seen in the study by Vetter and colleagues17). This might be explained by our block design; it may not have been sensitive to immediate amygdala activations, which usually decrease or habituate during presentation.
With respect to habitual emotion-regulation strategies, patients with ADHD and healthy controls differed significantly: in everyday life, people with ADHD use more suppression and healthy controls apply reappraisal more often. Expressive suppression is defined as hiding the expression of emotions when an emotional response has been induced. Thus, explicit instructions in an otherwise emotionally stable environment might have facilitated the application of reappraisal for ADHD patients in our study, a response that might not translate to emotion regulation in the real world, where emotional stimuli are often unexpected and induce rapid and habitual reactions. Interestingly, habitual reappraisal was positively correlated with ACC activation in patients with ADHD during the negative view condition. These data were in line with a previous study on habitual emotion regulation measured with the ERQ and PFC activation in healthy controls.60 These authors found no correlation between suppression and PFC activation during a cognitive control paradigm, but a positive correlation between habitual reappraisal and dorsal ACC activity.
The present findings were consistent with the results of our electrophysiological study of emotion regulation, in which patients with ADHD exhibited increased frontal late positive potential amplitudes during passive viewing of negative images and during emotion regulation.23 Interestingly, compared with healthy controls, a subgroup of medication-naive patients with ADHD exhibited larger centroparietal late positive potential amplitudes during reappraisal.
Taken together, both studies support the assumption that with explicit instructions to regulate emotions, patients with ADHD are quite competent and efficient in doing so. Both studies also support the proposition that emotional hyperresponsivity in ADHD patients induces stronger implicit emotion regulation.
Limitations
Some limitations of our study have to be considered. Participants were asked to withdraw from all stimulant medication at least 24 hours before scanning, which for the half-life of methylphenidate is a short time period. Also, we cannot rule out a long-term effect of stimulants. Unfortunately, the number of patients was too small to subdivide them into never-medicated and medicated groups. Additionally, the applied reappraisal paradigm might not relate very closely to everyday emotional events, which often happen without foreknowledge and which might be much more emotionally touching and disturbing. Although it may be difficult because any situation in the scanner is artificial, other reappraisal designs should be developed. Additionally, we investigated only negative emotional regulation, but some data show that patients with ADHD also have difficulty regulating positive emotional responses.61
Conclusion
The present findings have several translational implications. First, when thoroughly instructed, patients with ADHD exhibited behavioural and neural correlates of reappraisal similar to these correlates in healthy controls, although they tend to use suppression as an emotion-regulation strategy more often. Consequently, reappraisal training might be a useful module in therapy, which would aid patients in overriding dysfunctional emotion-regulation strategies. Additionally, emotional hyperresponsitivity, as implied by our data and previous studies, should be included in psychoeducational approaches, because awareness might help patients develop better coping strategies for intense emotional situations or stimuli.
Footnotes
Funding: This work was supported by the Deutsche Forschungsgemeinschaft (DFG, PE 1882/2-1). We acknowledge support from the Open Access Publication Fund of the University of Münster.
Competing interests: None declared.
Contributors: L. Materna, J. Trieloff, J. Bauer, A. Pedersen and P. Ohrmann designed the study. L. Materna, A. Shushakova, J. Trieloff, J. Bauer, A. Pedersen and P. Ohrmann acquired the data, which L. Materna, C. Wiesner, A. Shushakova, N. Weber, A. Engell, R. Schubotz, J. Bauer, A. Pedersen and P. Ohrmann analyzed. L. Materna, C. Wiesner, A. Engell, J. Bauer, A. Pedersen and P. Ohrmann wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
- Received August 13, 2018.
- Revision received December 5, 2018.
- Accepted January 14, 2019.