Abstract
Obesity is a major health challenge facing many people throughout the world. Increased consumption of palatable, high-caloric foods is one of the major drivers of obesity. Both orexigenic and anorexic states have been thoroughly reviewed elsewhere; here, we focus on the cognitive control of feeding in the context of obesity, and how the orbitofrontal cortex (OFC) is implicated, based on data from preclinical and clinical research. The OFC is important in decision-making and has been heavily researched in neuropsychiatric illnesses such as addiction and obsessive–compulsive disorder. However, activity in the OFC has only recently been described in research into food intake, obesity and eating disorders. The OFC integrates sensory modalities such as taste, smell and vision, and it has dense reciprocal projections into thalamic, midbrain and striatal regions to fine-tune decision-making. Thus, the OFC may be anatomically and functionally situated to play a critical role in the etiology and maintenance of excess feeding behaviour. We propose that the OFC serves as an integrative hub for orchestrating motivated feeding behaviour and suggest how its neurobiology and functional output might be altered in the obese state.
Introduction
The obesity pandemic is one of the biggest global health challenges. Obesity is a chronic illness that affects people regardless of gender, age, socioeconomic status or geographical location.1–3 Obesity is defined as a body mass index (BMI) greater than 30 kg/m2 and is associated with multiple comorbid diseases such as type 2 diabetes, stroke, cancer, depression and anxiety.1,4 Historically, obesity rates have been low;5 however, over the past 35 years, there has been a rapid increase in obesity rates and comorbid diseases.1–3,5,6 Although other environmental factors such as decreased energy expenditure and increased costs of healthy foods contribute to weight gain, multiple meta-analyses have concluded that overeating high-caloric food is the primary contributing factor to obesity.5–7 The modern obesogenic environment is largely related to the availability of easy-to-access, low-cost, highly palatable and energy-dense food, and this is a considerable driver of overeating.5,6 Prepackaged, convenient, ultra-processed foods play on our innate liking of sugars, salts and fats,8 and are often eaten even when energy requirements have been met.9 Understanding the neurobiological mechanisms of reward and influence of cognitive control that lead to excessive food intake may generate new knowledge about why we overeat despite satiety, and it may also point to potential therapeutic interventions.
Multiple brain regions and interacting systems regulate the orexigenic and anorexigenic states.10,11 The canonical understanding of food intake has been driven by a body of work describing how the adipocyte-released cytokine leptin regulates the neural control of energy balance through its modulation of the melanocortin system that originates in the arcuate nucleus of the hypothalamus.12 Leptin influences the alterations in excitability of these neurons that differentially drive food intake. Activation of neuropeptide Y/agouti-related peptide (AgRP) neurons drives food intake by AgRP-mediated inhibition of melanocortin 4 receptors. Activation of proopiomelanocortin/cocaine- and amphetamine-regulated transcript neurons releases α-melanocyte stimulating hormone, an agonist of melanocortin 4 receptors, to suppress food intake.13 The arcuate nucleus of the hypothalamus receives blood-borne and neuronal signals that assess energy status and modulate the neural activity of these cells to achieve energy balance.
Food is also consumed for reasons other than balancing energy needs, such as stress eating, social eating and eating for pleasure.14 Importantly, the hypothalamic, mesolimbic, subcortical and prefrontal regions all interact to drive food consumption (see reviews by Berthoud and Morrison,15 Kenny,16 and Timper and Brüning17). The mesolimbic dopamine system encodes cues that predict food availability and motivate people to engage in food-seeking.18 Access to palatable food can increase synaptic strength onto ventral tegmental area (VTA) dopamine neurons, an effect that drives increased food approach behaviours.19 In an environment with ready access to highly palatable, energy-dense food, the cognitive control of eating behaviour plays a dominant role in regulating body weight. For example, tasks involving response inhibition (such as the stop signal or Stroop tasks), decision-making tasks (such as the Iowa Gambling Task) and the relative reinforcing value of food task have a highly consistent relationship with BMI and eating behaviour, such that poorer performance on these tasks predicts higher BMI.20 Thus, brain circuits in the frontal cortex involved in response inhibition, decision-making and reward valuation also play key roles in modulating food intake. The role of the medial prefrontal cortex (mPFC) in feeding has been evaluated in other reviews,21 but the potential role of the orbitofrontal cortex (OFC) in ingestive behaviour and sensitivity to obesity has not received as much attention. This review will focus on the OFC and its role in obesity and feeding behaviour.
Anatomy and circuitry of the OFC in response to diet
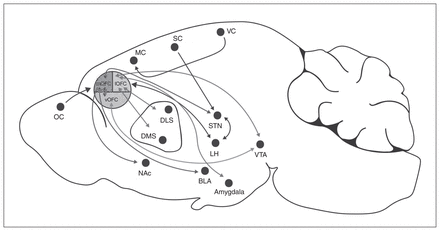
In rodents, the OFC is located dorsally to the rhinal sulcus and rostrally adjacent to the agranular insular areas. It can be subdivided into the ventral, ventrolateral, lateral, medial and dorsolateral anatomic distinctions based on relative location to the ventral midline (Fig. 1).22,23 In humans, there is some inconsistency in the anatomic borders of the OFC, but this structure can broadly be classified as Brodmann areas 10, 11 and 47.22,23 Subregions of the OFC in human and nonhuman primates are similar, but the rodent OFC does not contain a granular layer.24,25 In fact, the rodent OFC consists of agranular cortical regions, meaning that these regions lack a granular layer of small pyramid-shaped neurons in the middle cortical layers. The cortical column consists of layers I to VI. In layer II of the medial OFC, the cells are spread more homogeneously compared with the unevenly distributed layer II cells of the prelimbic regions of the mPFC, which has a densely packed layer I.26 Furthermore, the lateral OFC is easily differentiated between layers I and II/III in contrast to the ventrolateral OFC, which has an even distribution of pyramidal neurons.26
Afferent and efferent projections from subregions of the rodent orbitofrontal cortex. BLA = basolateral amygdala; DLS = dorsolateral striatum; DMS = dorsomedial striatum; LH = lateral hypothalamus; lOFC = lateral orbitofrontal cortex; MC = motor cortex; mOFC = medial orbitofrontal cortex; NAc = nucleus accumbens; OC = olfactory cortex; SC = somatosensory cortex; STN = subthalamic nuclei; VC = visual cortex; vOFC = ventral orbitofrontal cortex; VTA = ventral tegmental area.
Similar to other cortical regions, the OFC comprises inhibitory interneurons that synapse onto glutamatergic pyramidal neurons in layer II/III.27 These interneurons can be further classified as parvalbumin-positive (PV+), somatostatin, vasoactive intestinal polypeptide or cholecystokinin.28,29 The basket-cell morphology of these interneurons form vast perisomatic synapses.30 Basket cells are highly branched interneurons that form axosomatic synapses, and are therefore well positioned to coordinate pyramidal firing.31 Indeed, PV+ basket neurons entrain principal neuron output via γ-frequency oscillations (25–100 Hz).32 The PV+ interneurons in the OFC have been implicated in cognitive flexibility, such that transgenic mice with reduced PV expression showed impaired performance on a reversal learning task in concordance with altered OFC neuronal activity.33 Several studies have suggested that diet can also influence PV+ expression. In adult rats, exposure to a cafeteria diet for 6 weeks (ad libitum or intermittent access) did not alter PV+ expression in the lateral OFC.34 However, in mPFC subregions, intermittent access to a high-fat, high-sucrose diet during the adolescent period in rats (a manipulation that caused a small weight increase over controls) decreased PV+ interneuron expression in the infralimbic cortex.35,36 Intermittent access to 10% sucrose alone also decreased PV+ expression in the prelimbic cortex of adolescent male rats, but not the infralimbic cortex.37 This finding suggests that there may be differences in the influence of diet on PV+ expression in different cortical regions. Alternatively, the age of animals during diet exposure or duration of diet exposure may differentially influence PV+ expression.
Diet exposure can also influence γ-aminobutyric acid (GABA) release. An 8-week, high-fat diet decreased GABA in rat frontal cortex homogenates measured with high-performance liquid chromatography.38 In the lateral OFC, obesity — but not limited access to diet — decreased GABAergic release probability onto layer II/III pyramidal neurons as measured by a decrease in the frequency of GABAergic miniature release events, as well as a paired pulse facilitation in slice electrophysiology recordings.34 Because PV+ expression was not different in the lateral OFC of these obese rats, other interneurons or GABAergic inputs to lateral OFC pyramidal neurons may underlie decreased GABAergic synaptic transmission onto lateral OFC pyramidal neurons.34 Another proposed mechanism is that perineuronal nets influence GABAergic synaptic transmission. Perineuronal nets are extracellular matrix structures surrounding PV+ interneurons that can regulate synaptic plasticity.39 The expression of perineuronal nets can be influenced by diet. Exposure to a high-fat diet, independent of weight gain, decreased the number of perineuronal nets in the ventral OFC.40 Similarly, adolescent exposure to a high-fat, high-sucrose diet decreased expression of perineuronal nets in the infralimbic cortex of the mPFC.36 Taken together, GABAergic signalling in the OFC and mPFC may be especially sensitive to obesogenic diets and may influence cognitive flexibility.
The OFC pyramidal neurons also exhibit changes with an obesogenic diet. Decreases in basilar spines of lateral OFC pyramidal neurons have been found with an increase in branching of the basilar dendrites.34 In contrast, no changes were observed in apical spines or dendritic branching. Notably, basal and apical spine density is decreased in the prelimbic and infralimbic cortices after 3 weeks of exposure to a high-fat diet.41 Taken together, diet-induced obesity alters OFC cellular structure and function, and these changes may differ from those occurring in the mPFC.
Neural correlates of OFC function
The OFC encodes value in an identity-specific manner. This specificity ensures that a decrease in the value of food after a meal does not affect the value of other rewards, such as protection from predators or reproduction. For example, populations of OFC neurons initially respond to either sucrose (sweet taste) or quinone (bitter taste). As animals learn task-associated outcomes, these neurons fire in anticipation of the taste, and then to cues associated with the palatable or unpalatable food.42 Interestingly, these neurons continue to fire even after these contingencies are heavily learned43 and do not scale if the reward is delivered or withheld unexpectedly.44 Thus, OFC neurons appear to fire in response to representations of expected outcomes.42–45 This is consistent with OFC activation in response to anticipatory events of a preferred food.45,46 A recent study demonstrated that VTA-projecting OFC neurons had stable calcium activity recorded in individual neuronal clusters in response to food-predicting cues that lasted after extinction of the cue–reward association.47 When single OFC neurons that previously responded to presentation of caloric rewards were selectively stimulated, mice increased licking responses to sucrose.48 Interestingly, when single OFC neurons that respond to social reward were selectively stimulated, mice decreased licking for sucrose,48 suggesting that OFC neurons appear to maintain their activity-specific representations. In addition, OFC activation can scale with hunger49–51 and the pleasantness of food rewards.45 Increased in vivo neuronal activation in the OFC occurs when hungry compared to when sated in rats, monkeys and humans.49–52 Taken together, these findings show that OFC neuronal activity encodes representations of outcomes and their subjective value in an identity-specific manner. Thus, disruption of the OFC with obesity or other comorbid disorders may result in altered value representations of foods.
Afferent and efferent projections of the OFC
The OFC integrates afferent and efferent projections, including sensory, limbic and prelimbic regions, to guide decision-making associated with food intake. Afferent projections include sensory inputs from the gustatory, olfactory and visual cortices22,23 and use these multisensory modalities to modify behavioural output. In rodents, the olfactory bulb is located anterior to the OFC, and via the shortest pathway, pyramidal neurons in the OFC are 3 synapses distant from olfactory sensory neurons. This is unique, because other sensory modalities (such as visual or gustatory) pass through multiple relay centres before reaching the prefrontal cortex.53 The OFC receives gustatory cues, and it has been demonstrated that OFC neurons encode information about sweetness intensity.54 The OFC also responds to sensory characteristics of stimuli, including the flavour, appearance and texture of the reward.55
The OFC receives input from other parts of the prefrontal cortex, including motor and premotor regions, and from limbic projections, including the basolateral amygdala and the VTA.55–57 These inputs can segregate based on OFC subregion (Fig. 1). The medial OFC receives inputs from the thalamus, and the lateral OFC receives strong inputs from the amygdala. 27 The central zone of the lateral and ventral OFC receives dopaminergic input from the VTA to guide adaptive response to changing outcome value and prediction error learning.44 In vivo calcium imaging of OFC neurons projecting to the VTA demonstrated increased activity in relation to long-term cue–reward memory representations of reward-seeking behaviour.47 Furthermore, decreasing the activity of dorsal-striatum-projecting OFC neurons reduces compulsive-like behaviour in mice trained to press a lever for optogenetic stimulation of VTA dopaminergic neurons despite a shock punishment.58 These data suggest that reciprocal projections between the OFC and the VTA guide reward-seeking behaviour, and could be modulated by obesogenic diets to bias food intake.
The basolateral amygdala and OFC circuit share dense reciprocal projections that support outcome-guided behaviour. 55,56 Outcome-guided behaviour is the scaling of action based on the value of the outcome. For example, if the value of the reward decreases, action to receive that reward will also decrease. One of the primary roles of the basolateral amygdala is to assign positive or negative valence to stimuli by forming associations between neutral cues with awarding or aversive properties, helping to guide consumption.59 Thus, the OFC integrates sensory and limbic inputs to help optimize actions.
The OFC can be further subdivided into anterior and caudal areas, such that the anterior posterior OFC projects to the dorsolateral prefrontal cortex, insula and thalamus, whereas the caudal OFC projects to the thalamus and amygdala.43,60 The lateral OFC also sends strong projections to the dorsal medial and dorsal lateral striatum,58,61,62 whereas the medial OFC projects to the nucleus accumbens23 and the basolateral amygdala.43 The OFC concurrently projects to the lateral prefrontal cortex, which connects widely to motor and premotor areas63 that could be important in guiding outcome responses. 64 Thus, the OFC is positioned to influence a variety of functions, including decision-making and action selection of food and rewards.
OFC and reward-seeking behaviour in lean animals
Instrumental (operant) learning guides specific actions (e.g., lever presses) based on the rewarding consequence of the outcome (e.g., delivery of food in a better-than-expected context). This type of learning is based on flexible contingencies in which actions increase when a desirable outcome is to be achieved and decrease for a less desirable outcome or to avoid harmful and aversive outcomes.65 Goal-directed behaviour is a contingent relationship of value and associated outcome. It applies flexible learning, such that interaction is based on the reinforcing or aversive outcome value and is flexible in the face of changing contingencies. For example, goal-directed rodents will adapt their behaviour to maximize a desirable outcome. In contrast, habitual behaviour is insensitive to decreased current values of the outcome. For example, manipulation of the outcome (making the reward less valuable or even aversive) has no immediate effects on behaviour, such that animals continue to respond regardless of adverse or poorly optimized consequences.43,65 Appropriate decision-making requires integration of previous memories and outcomes from similar circumstances.43 The shift from goal-directed behaviour to habitual behaviour has been implicated in many disease states, including maladaptive decision-making, obsessive–compulsive disorder, eating disorders and addiction.66–68
The OFC is essential to the decision-making process, because it estimates the likelihood of a specific outcome to guide future responses. This estimation can be tested using outcome devaluation tasks. Outcome devaluation is a type of instrumental conditioning that can test goal-directed and habitual behaviour. Typically, animals will decrease a cueevoked response or action if the outcome (usually food) is devalued by satiation with the same type of food or by pairing the food with sickness using lithium chloride in a conditioned taste-aversion paradigm. Alternatively, the action to retrieve the reward (lever press) can be devalued by changing the contingency of the lever, so that action at the lever is no longer required to obtain a reward, and the animal must withhold response. This reversal of contingency requires the learning of new rules to obtain the reward. The knowledge that the OFC is critical in encoding the current value of rewards is supported by lesion and inactivation studies. Inactivation or lesions of the OFC after learning the cue association and before testing has been implicated in the devaluation of food rewards by sickness,69,70 satiety61,71,72 and contingency degradation tasks.73 The OFC has also been implicated in reversal learning,43,59 whereby animals must ignore their previously learned strategy and learn new rules to obtain a reward. 74 Finally, temporary inactivation of the OFC after training and stimulus-selective satiety before testing prevented accurate reward devaluation in a Pavlovian task.61 Based on these studies, it has been proposed that the OFC is responsible for updating the values of rewards and using this learned representation of outcomes to guide behaviour.59 Outcome expectancy relies on predictive cues that guide behaviour and the memory of what those cues predicted. With predictive cues guiding real-time behavioural computations and memory, the correct application of previous experiences can be applied to new situations.49 Outcome expectancy can provide information for real-time learning so that future behaviour is adaptive. Because outcome expectancy is often studied in the context of food and reward,57 it likely plays a key role in the adaptive regulation of food intake.
The OFC supports goal-directed behaviour, likely through its projections to the dorsomedial and dorsolateral striatum.61 The dorsomedial striatum has also been implicated in goal-directed behaviour: lesions in this brain region result in habitual behaviour.75–78 In comparison, the dorsolateral striatum modulates habitual behaviour: lesions in this brain region result in a shift to goal-directed behaviour.76–78 Conditioned taste aversion and selective satiety outcome devaluation are dependent on multiple brain structures, including the amygdala, the gustatory cortex and the OFC.43,79 Lesions in the gustatory cortex and amygdala inhibited both the acquisition and retrieval of outcome devaluation.79 In comparison, lesions in the OFC impaired cue-induced devaluation of sucrose when paired with lithium chloride, but not initial learning.71 Importantly, OFC lesions do not impair Pavlovian or instrumental learning; rather, acquisition of conditioned taste aversion is dependent on the basolateral amygdala, and choice decisions require the OFC.42,43 Consistent with this finding, pharmacological inactivation of the OFC impaired choice tests in outcome devaluation, but not the retrieval of the memory.69 Inactivation of the OFC does not change the perceived palatability of food rewards.80 Taken together, these findings show that the involvement of the OFC in outcome devaluation may serve to integrate reward-predictive cues and the new value of the rewards. In the context of feeding, the OFC integrates sensory information from consumption experience and performs computations to establish appropriate food-approach behaviour.
OFC and reward-seeking behaviour in obese animals
It has been proposed that goal-directed and flexible behaviour is impaired with obesity. Rats exposed to an unlimited cafeteria diet for 6 weeks81 experienced disruption in a Pavlovian devaluation task (Table 1). Rats were trained to associate 2 cues with distinct foods. Then, one of the foods was devalued with sensory-specific satiety. Rats on a cafeteria diet responded equally to both food predictive cues. The authors concluded that consumption of a cafeteria diet impaired stimulus–outcome learning and cued food associations.81 However, because cafeteria-fed rats decreased consumption of both valued and devalued food after selective satiation, others have argued that satiety-induced devaluation was not effective in selectively reducing the value of the pre-fed food in the cafeteria-fed rats; the deficit observed may not have reflected impaired stimulus–outcome learning, and instead may have reflected the insensitivity of the cafeteria-fed rats to selective satiety-induced devaluation.100 An alternate outcome devaluation method for testing this hypothesis would be to use a procedure that avoids reliance on satiety, such as lithium-chloride-induced devaluation (conditioned taste aversion).100 In another study, rats with 5 weeks of restricted access to sweetened condensed milk displayed a similar disruption in outcome devaluation, whereby rats with restricted access responded similarly to the undervalued and devalued reward conditions.99 Notably, rats with continuous access to the palatable food consumed fewer calories than those with restricted (binge-like) access but did not have deficits in outcome devaluation, suggesting a relationship with caloric intake and impairment in devaluation.99 Furthermore, in contrast to the findings of Reichelt and colleagues,81 the diet had no effect on sensory-specific satiety when the foods were freely available, suggesting that the deficit in reward devaluation in binge-access rats was associated with impaired stimulus–outcome learning rather than differences in satiety. 99 Rats exposed to a high-fat diet for 3 months also exhibited insensitivity to devaluation, but only if they were trained on a random interval schedule, whereby rewards were delivered in response to random numbers of lever presses that facilitated habitual response.101 These findings might suggest that energy-dense diets affect sensitivity to devaluation by facilitating a transition to inflexible response in situations employing behavioural strategies that are highly sensitive to outcome devaluation. In contrast to this idea, using an outcome devaluation task that discouraged habitual performance, whereby actions were performed without consideration of their consequences, rats given 6 weeks of exposure to a junk-food diet demonstrated impaired satiety-induced outcome devaluation.98 Thus, rats continued to demonstrate impairment in the selection between 2 distinct food-seeking actions when one of the food outcomes was devalued without the development of habit-like behaviour.
Comparison of human and rodent behavioural performance and alterations in OFC structure and function
Another method to test whether diet exposure impairs cognitive control over eating or alterations in stimulus–outcome learning is to use a Pavlovian Instrumental Transfer task.100,102 This task tests whether food-predicting cues can invigorate response for a cue associated with a specific food in rats exposed to control or palatable food diets.100,102 To further test for impairments in stimulus–outcome learning with junk-food diets, Kosheleff and colleagues98 tested the hypothesis that junk-food diets would impair control over specific food-seeking actions even when food values were not the primary basis for decision-making. Rats failed to use cue-elicited food expectations to guide their selection of actions based on the specific foods they represented in the Pavlovian Instrumental Transfer task. In other words, rats on the junk-food diet were impaired in selecting actions based on expected food value or the presence of food-paired cues,98 suggesting that energy-dense or palatable diets can disrupt cognitive control over feeding. Future work should test how the OFC is implicated in these diet-induced changes to flexible behaviour.
OFC and obesity in humans
Recent neuroimaging studies in humans are beginning to elucidate the complexity of the role of the OFC in the behavioural and neurobiological phenotypes that underlie obesity (Table 1). Structural and volumetric differences can be observed in the OFC related to body weight. People who are obese or morbidly obese have decreased OFC grey matter volume,82,83 decreased total OFC volume84 and altered fluid distribution in diffusion-weighted imaging.85 These structural differences have been implicated in altered executive function. 83 Functional imaging demonstrates that the OFC, as well as other structures involved in reward processing, are activated in response to food cues (see van der Laan and colleagues103), and people who are obese show increased blood oxygen-level-dependent (BOLD) activity in brain regions that encode cognitive control and reward (see Lowe and colleagues21). Obese individuals showed increased activation of the OFC in response to visual food cues than leaner individuals. 86 Interestingly, this activation persisted even when people were sated, suggesting that people who are obese appear to be more responsive to food cues when sated than lean individuals. Notably, increased BMI predicts OFC activation in response to food images.104,105 Consistent with this, obese women who fasted for 8 to 9 hours showed greater activation in the OFC, nucleus accumbens, anterior cingulate cortex and mPFC in response to images of highly palatable, high-calorie food compared with images of neutral or low-calorie food.87 It has been proposed that obesity may be linked to increased neural responses in reward anticipation from food cues, but decreased responses during food consumption. 88 Activation of the OFC in response to food cues in obese people regardless of satiety may underlie their vulnerability to overeating and diet failures.
Similar to rodents, the human OFC shows functional heterogeneity, such that the medial OFC encodes the value of food rewards, whereas the lateral OFC represents the nutritive encoding that is then integrated and processed in the medial OFC to compute overall value.106 Value encoding is the scaling of activation with the subjective value of the reward; this can be associated with food, monetary or other rewards. Nutritive encoding is activation based on perceived nutritive value, such that the subjective value of foods can be predicted by their perceived nutritive composition, including fat, carbohydrate, protein or vitamin content.106 Identity-specific goal representations in the lateral OFC can also be within a class of valued items. For example, in people who rated the value of 2 different foods as the same (e.g., milk-shake and chocolate), the lateral OFC encoded these foods differently.106,107 Consistent with this finding, functional MRI showed that pleasant tastes of glucose compared to unpleasant salt tastes activated different areas of the OFC, suggesting that the OFC encodes both positive and negative affective valence.51,108 The medial OFC encodes general reward value, such that food rated equally in value is encoded as equal representations. 109 For example, in people who rated 2 different foods as the same (e.g., milkshake and chocolate), the medial OFC encoded these values as the same.109 This anatomic differentiation may help fine-tune food value and reward-encoding. When hungry, lean individuals chose a high-intensity, palatable odour compared with a different low-intensity, value-matched odour after devaluation via consumption of that food, they preferred the high-intensity odour of the non-devalued food. This odour-specific satiety was represented via the lateral posterior OFC, where pattern-based changes were found in functional MRI signals toward the sated odour,110 demonstrating the role of the OFC in encoding flexible representations of rewards via devaluation.51 Similar to obese rodents, obese humans also have deficits in satiety-induced outcome devaluation: a higher BMI is associated with reduced reward devaluation.89 Future research should assess whether this impaired reward devaluation in obese people is identity-specific or a general devaluation of rewards. Taken together, these findings suggest that obesity is associated with heightened response in the OFC to food and food cues, as well as reduced reward devaluation, and thus may be implicated in impairments in flexible food representations and behaviour.
Executive function and obesity in humans
Neurocognitive tests can be correlated with weight gain and obesity, and many studies have tried to uncover the genetic111,112 and behavioural links that can lead to overeating.113 Self-reported impulsivity traits have been positively correlated with caloric consumption.114 Executive control can be broadly defined as flexible, goal-directed behaviour and can be further broken down into response inhibition, attention shifting and working memory. Multiple groups have reviewed the correlation between obesity and executive function (see Valnik and colleagues20 and Yang and colleagues113). Here, we will focus on OFC-dependent response inhibition tasks.
The OFC plays an important role in response inhibition in multiple tasks and disorders.22,115 In the Stroop task, participants must name the word colour while ignoring what word is written (example: “red” written in green ink). The OFC is activated during response inhibition,116 and manipulations of the OFC via direct-current transcranial stimulation improve performance on the Stroop task.117 Performance on the Stroop task was also correlated with BMI: people with a higher BMI showed decreased response inhibition,90 and better scores were associated with weight loss.118 Furthermore, an increase in BMI is associated with decreased prefrontal and cingulate gyrus metabolic activity, which in turn is associated with poorer Stroop task scores.91 In a go/no-go task modified to include food cues, obese people made more errors than lean controls,92 and this finding was correlated with reduced activation of the OFC during this task in obese people compared with lean controls.104 The OFC may be a critical area for neuromodulation or other therapeutic intervention to improve impulse control and cognitive flexibility in obese people.
OFC and binge-eating disorder
Binge-eating disorder is the most prevalent specific eating disorder, and it is associated with obesity, although many individuals with binge-eating disorder are not obese.119 Recent studies have compared brain structure, neurochemistry and function in people with binge-eating disorder and obesity and in people without obesity (reviewed in Balodis and colleagues120). In a preliminary report, obese people with binge-eating disorder showed increased BOLD activity in the OFC in response to pictures of binge-type foods compared to non-food stimuli (Table 1).93 This finding was consistent with those of other studies showing an increase in BOLD activity in the medial OFC in response to food cues and a positive association between the severity of binge-eating disorder and OFC activation.94,95 In these studies, the authors were unable to parse out differences in OFC activation between lean and obese people with binge-eating disorder, although changes in striatal function may distinguish obese people from those with binge-eating disorder.121 In other tasks, eating restraint in people with binge-eating disorder has been inversely associated with activity in the OFC, anterior cingulate cortex and ventral medial PFC during a food Stroop task.97 Binge-eating disorder and bulimia nervosa are associated with increased OFC and anterior cingulate cortex grey matter volume compared with lean controls.96 It is possible that alteration in function of the OFC in people with binge-eating disorder may underlie dysfunctions in food reward processing and/ or controlled regulation of food intake. Thus, the OFC may be a viable target for neuromodulation in treatment of eating disorders.
Conclusion
The OFC integrates sensory and limbic cues to help guide feeding behaviour.22 A wealth of evidence indicates that neurocognitive measures for impulse control, decision-making and reward valuation are associated with eating behaviour and BMI.20 Interestingly, some of the genetic factors associated with obesity may underlie different neurocognitive endophenotypes in obese people.111 Many of these neurocognitive endophenotypes, such as impaired cognitive control of response inhibition regarding food intake, are associated with OFC function,86,113 supporting the notion that the OFC is critically implicated in obesity. In rodent studies, palatable or energy-dense diets impair outcome devaluation, stimulus–response associations and the selection of actions based on expected food value or the presence of food-paired cues. Future work should focus on the impact of palatable, obesogenic diets on mechanistic changes in OFC function to further understand how the OFC adapts to the obese state and how this may lead to further food intake. Furthermore, the OFC may be a valid target for therapeutic intervention in people with obesity for weight loss or uncontrolled eating.
Footnotes
Competing interests: None declared.
Contributors: Both authors participated equally in the conception of the article and analysis and interpretation of the data. Both authors wrote and reviewed the article, approved the final version for publication and certify that no other individuals not listed as authors have made substantial contributions to the paper.
- Received September 25, 2019.
- Revision received November 30, 2019.
- Accepted December 9, 2019.