Abstract
Background: Treatment-resistant bipolar depression can be treated effectively using electroconvulsive therapy, but its use is limited because of stigma and cognitive adverse effects. Magnetic seizure therapy is a new convulsive therapy with promising early evidence of antidepressant effects and minimal cognitive adverse effects. However, there are no clinical trials of the efficacy and safety of magnetic seizure therapy for treatment-resistant bipolar depression.
Methods: Participants with treatment-resistant bipolar depression were treated with magnetic seizure therapy for up to 24 sessions or until remission. Magnetic seizure therapy was applied over the prefrontal cortex at high (100 Hz; n = 8), medium (50 or 60 Hz; n = 9) or low (25 Hz; n = 3) frequency, or over the vertex at high frequency (n = 6). The primary outcome measure was the 24-item Hamilton Rating Scale for Depression. Participants completed a comprehensive battery of neurocognitive tests.
Results: Twenty-six participants completed a minimally adequate trial of magnetic seizure therapy (i.e., ≥ 8 sessions), and 20 completed full treatment per protocol. Participants showed a significant reduction in scores on the Hamilton Rating Scale for Depression. Adequate trial completers had a remission rate of 23.1% and a response rate of 38.5%. Per-protocol completers had a remission rate of 30% and a response rate of 50%. Almost all cognitive measures remained stable, except for significantly worsened recall consistency on the autobiographical memory inventory.
Limitations: The open-label study design and modest sample size did not allow for comparisons between stimulation parameters.
Conclusion: In treatment-resistant bipolar depression, magnetic seizure therapy produced significant improvements in depression symptoms with minimal effects on cognitive performance. These promising results warrant further investigation with larger randomized clinical trials comparing magnetic seizure therapy to electroconvulsive therapy.
Clinical trial registration NCT01596608; clinicaltrials.gov
Introduction
Bipolar disorder is a recurrent neuropsychiatric disorder associated with substantial burden and impairment.1 It is characterized by episodes of mania or hypomania and episodes of depression, with the latter being the most burdensome.2 In patients with bipolar disorder, major depressive episodes exceed mania or hypomania episodes in frequency and duration, 3,4 and there is a significantly lower probability of recovery following a depressive episode than after a manic episode. 5 Compared with unipolar depression, treatment resistance in bipolar depression is 1.6 times higher and relapse after successful treatment is 3.4 times higher.6
Electroconvulsive therapy (ECT) is an effective treatment for bipolar disorder.7 Treatment guidelines for bipolar depression consistently recommend ECT in those who do not respond to first-line pharmacotherapy and those with a high risk of suicide or with severe symptoms, including catatonia or psychotic features.8 Electroconvulsive therapy is considered to be equally effective in both unipolar and bipolar depression; recent meta-analyses have estimated that remission rates exceeded 50%.9,10 A recent randomized clinical trial in patients with bipolar depression, ECT demonstrated superiority in response rates compared with algorithmbased pharmacotherapy.11 A recent large naturalistic study that included 295 patients with bipolar depression found a 68.1% response rate in those receiving ECT (with a minimum trial of ≥ 3 treatments), as measured by the Clinical Global Impression Improvement Subscale.12 However, use of ECT remains limited because of associated cognitive adverse effects (e.g., memory impairment)13 and the stigma associated with ECT.14
Magnetic seizure therapy (MST) is a newer noninvasive neuromodulation therapy that induces generalized seizures using high-frequency repetitive transcranial magnetic stimulation. 15 The generation of rapid time-varying magnetic fields creates electrical eddy currents that induce neuronal stimulation in the brain. Relative to ECT, MST is unaffected by impedance from the scalp or skull. As a result, there tends to be less stimulation of deeper brain structures (e.g., hippocampus) that are associated with memory function.16 Emerging clinical evidence supports the antidepressant effects of MST in treatment-resistant unipolar depression, and it may have efficacy comparable to ECT.17–19 As well, MST has been associated with shorter reorientation times and fewer cognitive adverse effects,20 suggesting that MST may be cognitively safer and better tolerated than ECT.
To date, studies of MST in treatment-resistant depression have focused on major depressive disorder,17,18 and only case studies have described the use of MST for bipolar disorder.21,22 Two cases of treatment-emergent mania have also been reported, 21 including in 1 patient with a diagnosis of bipolar disorder before MST. A small number of patients with bipolar disorder have also been included in randomized trials that compared MST and ECT.17,23 To date, however, no subgroup analyses or specific trials in patients with treatment-resistant bipolar depression (TRBD) have been published. Thus, we conducted an open-label clinical trial to evaluate the clinical efficacy and cognitive effects of MST in patients with TRBD. We explored prefrontal MST at 4 frequencies (100, 60, 50 and 25 Hz) and vertex at 100 Hz in sequential cohorts. The MST studies have most commonly used high-frequency 100 Hz stimulation;17,18,24 however, earlier studies of MST were conducted with medium-frequency ranges between 40 and 60 Hz.25,26 Furthermore, preclinical research has suggested that lower-frequency ranges may be more effective in seizure induction, with MST at approximately 22 Hz.27 Recently, we published the results of a large open-label clinical trial of MST in treatment-resistant major depressive disorder using the same protocol as the current study with low, medium or high frequencies and the stimulating coil placed over the prefrontal cortex.19 This was the first study of MST using a prefrontal placement; all previous MST studies have stimulated at the vertex. Because the pathophysiology of bipolar disorder is strongly associated with prefrontal cortex neurocircuitry,28 it is important to determine if there are advantages to stimulating over this area compared with the vertex. We hypothesized that MST would produce clinically significant response and remission rates in TRBD without any clinically meaningful changes in cognitive function.
Methods
Setting and participants
Patients with TRBD were recruited at the Centre for Addiction and Mental Health (CAMH), a large academic mental health hospital in Toronto, Ontario, Canada, that provides secondary and tertiary care to a large urban and suburban catchment area. The protocol was approved by the CAMH Research Ethics Board in accordance with the Declaration of Helsinki. All patients provided written informed consent. Patients were considered eligible if they had a DSM-IV diagnosis of a major depressive episode with or without psychotic features in the context of bipolar disorder based on the Structured Clinical Interview for the DSM-IV criteria;29 were referred for a course of ECT; were 18 to 85 years old; had a total score of ≥ 21 on the 24-item Hamilton Rating Scale for Depression (HRSD-24),30 indicating a moderate to severe symptom severity; and were on a medically acceptable form of birth control, if they were a woman of childbearing potential. Exclusion criteria included unstable medical or neurologic condition, or currently pregnant or lactating; not considered eligible for general anesthesia for any reason; having a cardiac pacemaker, cochlear implant, implanted electronic device or nonelectric metallic implant; taking a benzodiazepine medication at a dose greater than lorazepam 2 mg/d or equivalent; taking any anticonvulsant medication; active substance misuse or dependence within the preceding 3 months; a diagnosis of delirium, dementia or a cognitive disorder secondary to a medical condition; a lifetime diagnosis of an eating disorder; another significant neuropsychiatric comorbidity; any history of suicide attempts within the preceding 6 months; or diagnosed with antisocial or borderline personality disorder as confirmed by the Structured Clinical Interview for DSM-IV-TR Axis II Personality Disorders.31 Depressed patients were treated at the Temerty Centre for Therapeutic Brain Intervention at CAMH. The current report focuses on patients with bipolar disorder, from a broader exploratory clinical trial across several psychiatric disorders (clinicaltrials.gov; NCT01596608). If participants were receiving pharmacotherapy for their current depressive episode at the time of enrolment, they were allowed to continue and instructed not to make any changes for the duration of their participation in the trial. Information on current medication usage was collected at baseline and after every 3 MST treatments to monitor for any changes.
Clinical assessment
Demographic information and clinical characteristics were collected from all participants at baseline following a clinical interview, including the duration of the current episode, years since first diagnosis, the number of previous mood episodes, and current and previous antidepressant treatment. Baseline medical comorbidity was assessed using the Cumulative Illness Rating Scale.32 Treatment resistance was quantified at baseline with the Antidepressant Treatment History Form (ATHF), modified to include medications specifically indicated for bipolar disorder.33 Each anti-depressant trial was rated on a scale of 0 to 5 based on the adequacy of the dose and the length of the trial. We assessed both the number of adequate medication trials (i.e., trials rated 3 to 5 on the ATHF) and the cumulative ATHF score, derived by adding the scores of all medication trials during the current episode.
The primary outcome measure was depression score on the HRSD-24. Symptoms were assessed at baseline, after every 3 sessions and at the end of treatment. Remission of depression was defined as a total score on the HRSD-24 of 10 or less, and a 60% decrease or more in scores from baseline on 2 consecutive ratings; response was defined as a 50% reduction or more on the HRSD-24 on 2 consecutive ratings. The emergence of mania was assessed after every 3 MST sessions using the Young Mania Rating Scale.34
Neurocognitive assessment
We conducted comprehensive neurocognitive assessment before and after MST. Pre- and post-treatment measures consisted of the Autobiographical Memory Interview–Short Form (AMI-SF),35 the MATRICS Consensus Cognitive Battery (includes the Brief Assessment of Cognition in Schizophrenia–Symbol Coding, Trail Making Test A, the Hopkins Verbal Learning Test–Revised, the Wechsler Memory Scale–Spatial Span, Category Fluency Animal Naming, Letter Number Span, Brief Visuospatial Memory Test–Revised and Neuropsychological Assessment Battery–Mazes),36 the Trail Making Test B,37 the Stroop Color and Word Test38 and the Controlled Oral Word Association Test.39 We also administered the Montreal Cognitive Assessment40 at baseline, after every 6 sessions and at the end of treatment, and we measured the time to reorientation after each MST session, defining reorientation as providing correct personal name, date, age, place, day of the week and date of birth. Neurocognitive data were unadjusted to normative scaled scores.
MST treatments
Magnetic seizure therapy was delivered 2 to 3 times per week using the MagVenture (MagPro MST) with a twin coil. A full treatment course was a maximum of 24 sessions or until the patient achieved remission of depressive symptoms. Placement of the twin coil was over the bilateral prefrontal cortex or the vertex. Stimulation trains were provided at 100% machine output. Stimulation frequency was determined at baseline and fixed for the duration of treatment at 100, 60, 50 or 25 Hz. Coil placement and stimulation frequency were evaluated in sequential order in 4 cohorts: prefrontal placement, high frequency; prefrontal placement, medium frequency; prefrontal placement, low frequency; and vertex placement, high frequency. Prefrontal placement was over F3 and F4 according to the international 10–20 system. An additional 6 participants had stimulation over the vertex, and placement was midline between the nasion and inion. At initiation of MST, the MST parameters were titrated to identify the lowest energy needed to reliably produce a seizure (i.e., seizure threshold). A maximum of 3 stimulations were provided per treatment session, and if a seizure was not reached, then titration continued at the next session. For a stimulation frequency of 50, 60 or 100 Hz, titration began with 2 seconds and increased in increments of 2 seconds up to a maximum of 10 seconds (for a total of 1000 pulses). For 25 Hz settings, titration began with 4 seconds and increased in increments of 4 seconds, with a maximum of 500 pulses. After the seizure threshold was identified, stimulations were administered with a duration 3 times longer than the initial threshold duration. If the maximum duration was reached, a second stimulation using the same parameters was given during the same session. Participants who did not have a seizure during 3 consecutive sessions were discontinued from the study. Symptoms of depression were assessed using the HRSD-24 after every 3 treatment sessions, and if the participant had not reached the predetermined remission criteria, the MST dose was increased at the next treatment. Participants continued treatment until they achieved remission or completed a maximum of 24 MST sessions. An adequate trial of MST was considered to be 8 or more treatments received.41
Anesthesia
Treatment was given under general anesthesia using methohexital sodium (0.375–0.75 mg/kg IV) and succinylcholine chloride (0.5–1.0 mg/kg IV) for neuromuscular blockade. If obtaining an adequate seizure was difficult, the dose of methohexital was decreased by approximately 50% and remifentanil (1.0–1.5 μg/kg) was added for additional sedation. Blood pressure, oxygen saturation, heart rate and electrocardiography were monitored throughout the MST procedure.
Statistical analysis
Statistical analysis was performed using SPSS 24 (IBM Inc.). Participants who completed an adequate course of MST were included in the analysis. We also included a per-protocol analysis that included all participants who completed a full course of MST (see above; defined as attainment of remission or a maximum of 24 treatments). Because the data were non-normally distributed, we compared pre- and postclinical and cognitive assessments using the Wilcoxon signed-rank test, a nonparametric statistical test. We calculated effect sizes using Cohen’s d and corresponding 95% confidence intervals. Tests were 2-tailed, and significance was set at α = 0.05 for the primary outcome, except for the neurocognitive measures, for which significance was set at α = 0.005 to minimize false-positive findings, given the 24 comparisons performed. We repeated the analyses using paired-sample t tests to corroborate the findings.
Results
Participant flow and sample characteristics
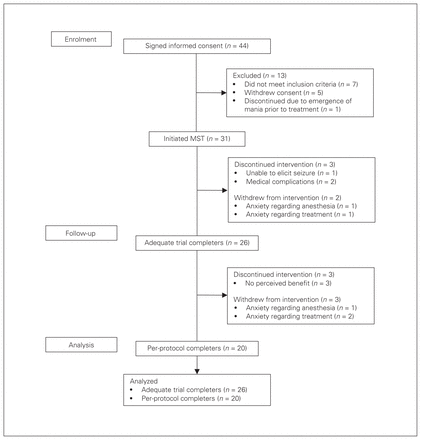
Of the 44 patients who consented to participate in the study, 7 did not meet the inclusion criteria, 1 discontinued because of the emergence of mania before treatment initiation and 5 withdrew consent. Thirty-one patients with bipolar disorder were enrolled in the study and received MST treatment; 26 received an adequate trial of treatment, defined as a minimum of 8 sessions. These participants were included in the primary analysis (Fig. 1, CONSORT diagram). Two patients withdrew before completing an adequate course of treatment because of an inability to tolerate the treatment as a result of discomfort with anesthesia (100 Hz prefrontal) and nausea (60 Hz frontal). Three others discontinued treatment: 1 participant was unable to adequately elicit seizures (100 Hz prefrontal), and 2 participants had medical complications unrelated to the treatment (1 investigated for possible pericarditis with inconclusive ultrasound findings and spontaneous remission of chest symptoms at follow-up; and 1 with myocardial infarction on the day after the third MST session). Baseline clinical and demographic variables are described in Table 1. In general, the sample was highly treatment-resistant to standard pharmacotherapy.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram for participant enrolment and dropouts.
Participant characteristics
Of the 26 patients who completed a minimally adequate trial of 8 MST sessions or more, 8 received prefrontal-placement high-frequency stimulation; 9 received prefrontal-placement medium-frequency stimulation; 3 received prefrontal-placement low-frequency stimulation; and 6 received vertex-placement high-frequency stimulation. From this group, 2 patients withdrew because of anxiety about the treatment (both 100 Hz prefrontal), 1 withdrew because of anxiety about anesthesia (50 Hz prefrontal) and 3 were discontinued because of no perceived benefit (100 Hz prefrontal, 50 Hz prefrontal and 25 Hz prefrontal). Twenty patients completed the per-protocol MST trial (a maximum of 24 treatments or remission on the HRSD-24): 5 received prefrontal-placement high-frequency stimulation; 7 received prefrontal-placement medium-frequency stimulation; 2 received prefrontal-placement low-frequency stimulation; and 6 received vertex-placement high-frequency stimulation. No statistical analyses were computed to evaluate possible differences associated with different stimulation frequencies and coil placements because of the small sample size of each subgroup.
In adequate trial completers, the mean number (± standard deviation [SD]) of adequate medication trials was 2.36 ± 1.50 for the current depressive episode. Data for medication trials were not available for 1 participant. The cumulative ATHF score mean (± SD) was 12.5 ± 8.41 for the current episode. In per-protocol completers, the number of adequate medication trials was 2.25 ± 1.48 and the cumulative ATHF score was 11.35 ± 7.43.
EEG seizure duration
We calculated the EEG seizure duration as the duration of the seizure on EEG averaged between the second and last session. Mean seizure duration (± SD) in adequate trial completers was 44.12 ± 21.99 seconds, and in per-protocol completers was 45.20 ± 23.48 seconds.
Depression outcomes
Adequate trial completers
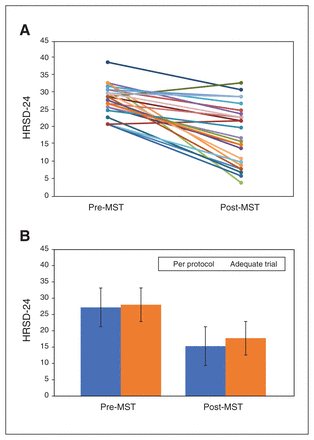
Among the minimally adequate trial completers, remission and response rates were 23.1% and 38.5%, respectively (Fig. 2A). For the overall group, we found a statistically significant reduction in HRSD-24 total score from baseline to the end of treatment (Z = −4.307, p < 0.001; Fig. 2B). The corresponding effect size for the level of change from baseline to the end of treatment was large (d = 1.25; 95% CI 0.42–1.57). Mean (± SD) HRSD-24 scores were 28.08 ± 4.41 at baseline and 17.77 ± 8.48 at the end of treatment.
(A) Profile plot for individual participants (n = 26) who completed an adequate trial of magnetic seizure therapy (MST; ≥ 8 sessions) on the 24-item Hamilton Rating Scale for Depression (HRSD-24). (B) Group differences on the HRSD-24 pre- and post-MST for participants who completed an adequate trial of MST (n = 26) and those who completed the entire course of treatment (up to 24 sessions or to remission; n = 20).
By treatment subtype, response and remission rates, respectively, were as follows: 100 Hz prefrontal, 37.5% and 37.5% (n = 8); 60 Hz prefrontal, 57.1% and 14.3% (n = 7); 50 Hz prefrontal, 0% and 0% (n = 2); 25 Hz prefrontal, 66.7% and 66.7% (n = 3); and 100 Hz vertex, 16.7% and 0% (n = 6).
Per-protocol completers
Among the per-protocol completers, the remission and response rates were 30% and 50%, respectively. From baseline to the end of treatment, we found a statistically significant decrease in HRSD-24 total score (Z = −3.866; p < 0.001), and the effect size was large (d = 1.61; 95% CI 0.62–1.98). Mean (± SD) HRSD-24 scores were 27.25 ± 4.17 at baseline and 15.35 ± 7.50 at the end of treatment.
By treatment subtype, response and remission rates, respectively, were as follows: 100 Hz prefrontal, 60% and 60% (n = 5); 60 Hz prefrontal, 57.1% and 14.3% (n = 7); 25 Hz prefrontal, 100% and 100% (n = 2); and 100 Hz vertex, 16.7% and 0% (n = 6).
Number of sessions required to achieve response or remission
For both the adequate trial and per-protocol groups, the mean (± SD) number of treatments to reach response criteria was 17.4 ± 5.80 and 17.5 ± 6.95, respectively. The smallest number of sessions required to achieve response or remission was 9, and the largest was 24.
Time to reorientation
Reorientation time was calculated as the mean ± SD in seconds between the second MST treatment and the last MST treatment. For adequate trial completers, the average time to reorientation was 8.51 ± 4.79 minutes. For per-protocol completers, the average time to reorientation was 8.76 ± 4.52 minutes.
Neurocognitive outcomes
Adequate trial completers
We assessed neurocognitive effects using 24 different neurocognitive tests before and after the MST course. We found no significant differences pre- and post-MST for 23 of the 24 scores (Appendix 1, Table S1, available at jpn.ca/190098-a1). We did find a statistically significant mean decrease of 18.9% from baseline to the end of treatment in total score on the AMI- SF (Z = −4.291, p < 0.001). We found the same result for both nonparametric and parametric analyses. Pre- and post-MST effect sizes for all cognitive measures are provided in Figure 3, showing no significant changes for the other 23 tests.
Effect sizes for cognitive outcomes presented with Cohen’s d and 95% confidence intervals. AMI-SF = Autobiographical Memory Interview–Short Form; BVMT-R = Brief Visuospatial Memory Test–Revised; Categories–animals = Category Fluency Animal Naming; COWAT = Controlled Oral Word Association Test; HVLT-R = Hopkins Verbal Learning Test–Revised; LNS = Letter Number Span; Mazes = Neuropsychological Assessment Battery–Mazes; MoCA = Montreal Cognitive Assessment; SS forward/ back = Weschler Memory Scale–Spatial Span; Stroop = Stroop Color and Word Test; Symbol coding = Brief Assessment of Cognition in Schizophrenia–Symbol Coding; Trails A/B = Trail Making Test, A or B.
Per-protocol completers
Participants who completed the trial per protocol had a statistically significant mean decrease of 17.8% from baseline to the end of treatment in total score on the AMI-SF (Z = −3.925, p < 0.001). We found no statistically significant differences from baseline to the end of treatment any of the other neuro-cognitive variables (Appendix 1, Table S2).
Safety
Among the 31 study participants who initiated MST treatment, 4 serious adverse events occurred; 2 were considered to be possibly related to MST. Several days after completing the trial per-protocol, 1 participant had a hypomanic episode that resolved quickly with an increase in medication. No other participants experienced hypomania or mania as identified clinically or with the Young Mania Rating Scale. Another participant was hospitalized because of a fall and a dislocated shoulder; after stabilization, this participant resumed the trial.
Two other serious adverse events were deemed unrelated to the intervention: 2 patients were discontinued from the study before completing an adequate course because of medical complications. One participant was hospitalized for a gallbladder removal. Another patient was admitted to hospital for a myocardial infarction the day after the third treatment (100 Hz prefrontal). Before MST treatment, this patient was identified as having a pre-existing heart condition but was medically cleared for participation in the trial. After the event, the impression from cardiology was that the MST treatment was unrelated to the chest pain the patient experienced.
Discussion
This open-label, nonrandomized clinical trial suggests that MST can be an effective and safe treatment for TRBD. Minimally adequate trial completers experienced a large and significant reduction in their depressive symptoms, resulting in a 38.5% response rate and 23.1% remission rate. The effect size was larger in the group that completed a full course of MST, who showed response and remission rates of 50% and 30%, respectively. Moreover, MST was found to have cognitive safety.
Magnetic seizure therapy is most commonly compared with ECT, which remains the standard noninvasive neuromodulation antidepressant treatment indicated for TRBD, and has high response and remission rates and quick speed of response. In a meta-analysis of 19 studies that compared unipolar to bipolar depression, including 9 prospective trials, the pooled remission rate for ECT was 52.3%.10 The remission rate in our study was somewhat lower, but it needs to be considered in the context of the cognitive adverse effects that were not addressed in the meta-analysis. A recent randomized controlled trial of algorithm-based pharmacotherapy versus right unilateral brief pulse ECT in patients with bipolar depression demonstrated superiority of ECT relative to pharmacotherapy for response rates (73.9% v. 35.0%, respectively) but not for remission rates (34.8% v. 30.0%, respectively).11 Speed of response varies in ECT depending on the electrode placement and pulse width; the greatest number of treatments is required for right unilateral placement and ultra-brief pulse width.42,43 In studies of right unilateral ultra-brief-pulse ECT that included patients with bipolar depression, the mean number of treatments to achieve response was approximately 12,44,45 which was comparable to the current study but some-what faster in achieving remission. Specifically, an average of 17 MST sessions was needed to achieve response, which represented a higher number of treatments and a lengthier treatment course relative to ECT.46 However, ultra-brief pulse width stimulation relative to brief pulse width, combined with right unilateral electrode configuration, has a slower speed of response in conjunction with better cognitive outcomes. 43 There may be a trade-off of a longer course of treatment for less cognitive impairment in both ultra-brief ECT and MST.
Our study also suggests that MST is well tolerated and has minimal effects on most cognitive functions. We found a relatively short time to reorientation with MST: adequate trial and per-protocol completers had mean (± SD) reorientation times of 8.51 ± 4.79 and 8.76 ± 4.52 minutes, respectively. Our findings were consistent with multiple previous studies that found quicker time to reorientation with MST relative to ECT.23,26,47 Time to reorientation following ECT has been reported to be 26 minutes 35 seconds,47 18 minutes26 and 8 minutes 21 seconds.23
Electroconvulsive therapy is known to produce cognitive adverse effects: this has been consistently demonstrated using objective cognitive measures,13 and in subjective reports from patients who described these cognitive effects as a deterrent to initiating or completing ECT therapy. Our study findings of adverse cognitive effects after ECT were in line with other studies that have used similar neurocognitive measures (e.g., Trail Making Test, Hopkins Verbal Learning Test–Revised) and found adverse effects for cognitive domains such as complex visual scanning and cognitive flexibility, attention, and verbal learning and memory.48–50 Historically, these cognitive adverse effects and patient self-report of cognitive concerns have contributed to the stigma associated with ECT.51,52
In unipolar depression, research to date has demonstrated that MST is cognitively safe, and relative to all standard methods of ECT delivery has a superior cognitive safety profile. 17,20 This study adds to a growing body of evidence that substantiates the neurocognitive safety of MST, because no neurocognitive test except for the AMI-SF showed a significant change after MST. The AMI-SF, which measures consistency in autobiographical recall from baseline to the end of treatment, showed a significant decrease in total score after MST. To complete the AMI-SF, the participant must recall the exact information provided to a question at baseline after the treatment course, and any variation in response is considered to be a memory error. As such, the score can only remain stable or decrease; it can never improve. Although our results may indicate a potential adverse effect of MST on this domain of autobiographical recall consistency, it is more likely that the effect we observed was associated with the passage of time, rather than with MST. Over the span of 3 months, the consistency of autobiographical memory recall is expected to decrease 28% to 40%, even in healthy, nonclinical samples.53 Similarly, in a study by Kessler and colleagues54 that compared the AMI-SF in patients with bipolar disorder who were treated with right unilateral brief pulse ECT or pharmaco-therapy, ECT was associated with a larger decrease in AMI- SF scores, but the pharmacotherapy group showed a 19.2% consistency loss, most likely related to an effect of time. The consistency loss in the pharmacotherapy group was similar to the decreased AMI-SF total score in our study with MST (18.9%), which was also less of a decrease than observed in the ECT group of the study by Kessler and colleagues (27.1%).54 Future studies are warranted to further assess this finding and would benefit from the inclusion of a comparator group to clarify the effects of time and treatment.
Limitations
Clear limitations of the present study were its small sample size, its open-label design and the lack of a comparator group. Furthermore, this study had limited power to analyze the impact of MST parameters, including stimulation frequency and coil placement. Because of the small sample size of the subgroups, we were unable to make meaningful interpretations of the different remission and response rates related to frequency and placement. Our recent study of MST in major depressive disorder with a much larger sample size showed that high-frequency (100 Hz) stimulation had higher antidepressant effects than moderate or low frequencies,19 and it will be important for future research to determine whether this is also the case for patients with bipolar disorder.
Conclusion
Our results showed that MST is an effective treatment for TRBD, with minimal adverse effects on cognitive function. To our knowledge, this is the first report of MST specifically in patients with TRBD, and further research is required to replicate and confirm these findings. A future randomized controlled trial of ECT and MST in patients with TRBD is now needed, with a larger sample and blinding to assessment and treatment allocation. If the efficacy of MST and ECT are comparable and MST is better tolerated, MST may advance the standard of care for patients with TRBD.
Footnotes
Competing interests: D. Blumberger reports grants from Magventure during the conduct of the study; other funding from Brainsway; and grants from the National Institute of Mental Health, the Canadian Institutes of Health Research, Brain Canada and the Patient Centred Outcomes Research Institute, outside the submitted work. S. McClintock reports grants from the National Institutes of Health and personal fees from Pearson Assessment during the conduct of the study. D. Voineskos reports grants from the Ontario Mental Health Foundation and the Innovation Fund of the Alternate Funding Plan for the Academic Health Sciences Centres of Ontario, outside the submitted work. J. Downar reports personal fees from Restorative Brain Clinics, TMS Neuro Health and Neurostim TMS Clinics, outside the submitted work. B. Mulsant reports grants from Brain Canada, the Canadian Institutes of Health Research, the Centre for Addiction and Mental Health Foundation, the Patient-Centred Outcomes Research Institute and the United States National Institutes of Health; and non-financial support from Eli Lilly, Pfizer, Capital Solutions Design LLC, HAPPYneuron and General Electric, outside the submitted work. P. Fitzgerald is supported by a National Health and Medical Research Council Practitioner Fellowship (1078567) and has received equipment for research from MagVenture A/S, Medtronic Ltd, Neuronetics and Brainsway Ltd. and funding for research from Neuronetics; he is on scientific advisory boards for Bionomics Ltd and LivaNova and is a founder of TMS Clinics Australia. No other competing interests were declared.
Contributors: D. Blumberger, P. Fitzgerald and Z. Daskalakis designed the study. D. Blumberger, J. Dimitrova, A. Throop, D. Voineskos and Z. Daskalakis acquired the data, which V. Tang, D. Blumberger, J. Dimitrova, S. McClintock, J. Downer, Y. Knyahnytska, B. Mulsant and Z. Daskalakis analyzed. V. Tang, Y. Knyahnytska and Z. Daskalakis wrote the article, which D. Blumberger, J. Dimitrova, A. Throop, S. McClintock, D. Voineskos, J. Downar, Y. Knyahnytska, B. Mulsant, P. Fitzgerald and Z. Daskalakis reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
- Received May 14, 2019.
- Revision received September 21, 2019.
- Accepted October 18, 2019.