Abstract
Background: Schizophrenia and bipolar disorder have been linked to alterations in the functional activity and grey matter volume of some brain areas, reflected in impaired regional homogeneity and aberrant voxel-based morphometry. However, because of variable findings and methods used across studies, identifying patterns of brain alteration in schizophrenia and bipolar disorder has been difficult.
Methods: We conducted a meta-analysis of differences in regional homogeneity and voxel-based morphometry between patients and healthy controls for schizophrenia and bipolar disorder separately, using seed-based d mapping.
Results: We included 45 publications on regional homogeneity (26 in schizophrenia and 19 in bipolar disorder) and 190 publications on voxel-based morphometry (120 in schizophrenia and 70 in bipolar disorder). Patients with schizophrenia showed increased regional homogeneity in the frontal cortex and striatum and the supplementary motor area; they showed decreased regional homogeneity in the insula, primary sensory cortex (visual and auditory cortices) and sensorimotor cortex. Patients with bipolar disorder showed increased regional homogeneity in the frontal cortex and striatum; they showed decreased regional homogeneity in the insula. Patients with schizophrenia showed decreased grey matter volume in the superior temporal gyrus, inferior frontal gyrus, cingulate cortex and cerebellum. Patients with bipolar disorder showed decreased grey matter volume in the insula, cingulate cortex, frontal cortex and thalamus. Overlap analysis showed that patients with schizophrenia displayed decreased regional homogeneity and grey matter volume in the left insula and left superior temporal gyrus; patients with bipolar disorder displayed decreased regional homogeneity and grey matter volume in the left insula.
Limitations: The small sample size for our subgroup analysis (unmedicated versus medicated patients and substantial heterogeneity in the results for some regions could limit the interpretability and generalizability of the results.
Conclusion: Patients with schizophrenia and bipolar disorder shared a common pattern of regional functional and structural alterations in the insula and frontal cortex. Patients with schizophrenia showed more widespread functional and structural impairment, most prominently in the primary sensory motor areas.
Introduction
Schizophrenia and bipolar disorder are severe and highly complex mental disorders with remarkably heterogeneous symptoms characterized by combinations of dysfunction in thoughts, perceptions, emotions and behaviour.1 Distinctions between schizophrenia and bipolar disorder have been challenged by recent epidemiological and biological studies.2,3 As well, shared clinical, cognitive and genetic features support an overlap across the apparent dichotomy.4–6 Clinically, accurately diagnosing and distinguishing between the 2 diseases is an urgent issue to provide appropriate treatment and improve patient outcomes, because they sometimes present with similar pathologies. Brain imaging could help to inform the debate by describing similarities and differences between these 2 disorders.
Resting-state functional MRI (fMRI), a noninvasive neuroimaging technique based on blood oxygen level dependency, has proven useful for investigating the pathophysiological mechanisms of mental illness.7 However, most analytic techniques for resting-state fMRI (such as functional connectivity, graph theory and independent component analysis) characterize the function of brain networks; local activity cannot be fully addressed using these approaches. Regional homogeneity can reflect the local temporal homogeneity of regional blood-oxygen-level-dependent signalling, and it has high test–retest reliability.8,9
Regional homogeneity is a voxel-based measure of brain activity that is conducted by calculating the Kendall coefficient of concordance of the time series of a given voxel in relation to those of its nearest neighbours (26 voxels) in a voxel-wise analysis.10 Increases and decreases in regional homogeneity values reflect aberrant neural activity in the brain, as well as the severity of affective symptoms.11 Regional homogeneity may be helpful for understanding the level of coordination of regional neural activity in the resting state and for revealing pathophysiological changes in mental disorders such as schizophrenia12–16 and bipolar disorder.17–20
Several studies have reported extensive neural activity changes in multiple brain regions in schizophrenia and bipolar disorder, including the frontal region, the temporal region, the parietal lobe and the limbic system,12,15,19–25 but these results were divergent. Some of the inconsistent findings might have been related to the low statistical power of individual studies, because most of the available studies had small sample sizes. A meta-analysis could help to increase statistical power and provide an estimate of the level of difference in regional homogeneity in schizophrenia and bipolar disorder.
As well as alterations in functional activity, differences in structural markers such as grey matter volume are also important because they may be relatively stable and serve as a foundation for functional neural activity. Voxel-based morphometry is an objective, automatic method of whole-brain analysis that allows for the detection of subtle morphometric intersubject differences in brain structure. Structural MRI studies have reported reduced grey matter volume using voxel-based morphometry analysis in multiple brain regions, including the frontal region, the temporal region, the parietal lobe and the limbic system in patients with schizophrenia12,15,19–25 and bipolar disorder.26–29 Shrinkage of neuropil, reduced neuron size and cell loss are thought to be possible underlying causes.30 These anatomic changes are believed to be a potential substrate of the altered integration of information in neocortical systems, and of symptom expression. However, most studies have used structural MRI to investigate schizophrenia and bipolar disorder separately. More effort should be made to integrate structural and functional imaging modalities to explore neuropathological mechanisms in these 2 disorders.
The aim of this study was to perform a quantitative, voxel-based meta-analysis of changes in regional homogeneity and grey matter volume in schizophrenia and bipolar disorder by taking advantage of the whole-brain resting-state fMRI and voxel-based morphometry studies published in recent years. We hypothesized that patients with schizophrenia and bipolar disorder would show common and specific patterns in local intrinsic neural activity and structural alterations.
Methods
Data sources, study selection and quality assessment
We conducted a comprehensive search of studies published between Jan. 1, 2000, and Nov. 28, 2020, using the databases of PubMed, Web of Science, Embase, SinoMed, Chinese National Knowledge Infrastructure and WanFang, and the following medical subject heading (MeSH) keywords: (rest OR resting) AND (schizophrenias OR schizophrenic disorders OR disorder, schizophrenic OR disorders, schizophrenic OR schizophrenic disorder) AND (ReHo OR regional homogeneity)/(voxel-based morphometry OR VBM). We then conducted another search using the following keywords: (ReHo OR regional homogeneity)/(voxel-based morphometry OR VBM) AND (bipolar disorder OR bipolar disorders OR manic-depressive psychosis OR manic depressive psychosis OR bipolar affective psychosis OR manic-depressive psychoses OR mania OR manic state OR manic states OR bipolar depression OR manic disorder OR manic disorders OR bipolar euthymic). We checked the reference lists of included studies and relevant review articles to identify additional relevant studies.
Studies that satisfied the following conditions were included in the meta-analysis: patients had been diagnosed with schizophrenia (including first-episode and chronic schizophrenia) or bipolar disorder (bipolar disorder I or bipolar disorder II) between the ages of 18 and 60 years; the study compared regional homogeneity or voxel-based morphometry in patients with schizophrenia or bipolar disorder versus healthy controls; 3-dimensional coordinates (Talairach or Montreal Neurological Institute) were reported for whole-brain regional homogeneity or voxel-based morphometry analysis; significant results were reported using thresholds for significance that were corrected for multiple comparisons or uncorrected with spatial extent thresholds; and the study was published as an original article (not as a letter or an abstract) in a peer-reviewed English- or Chinese-language journal.
Data sets were excluded for the following reasons: data were unavailable (e.g., missing neuroanatomical coordinates) even after contacting the authors by email or telephone; the study used dynamic regional homogeneity to compare patients versus healthy controls; or the study used a region-of-interest approach. For longitudinal studies we included only baseline data. In cases where patient data sets overlapped between separate articles, we included only the data set with the largest sample size and the most comprehensive information.
We used a 10-point checklist that has been used in previous meta-analyses of resting-state fMRI studies to assess the quality of each selected study (Appendix 1, Table S1, available at www.jpn.ca/lookup/doi/10.1503/jpn.210111/tab-related-content).31,32 We also reviewed the quality assessment of regional homogeneity studies based on the recommendations of Iwabuchi and colleagues,33 and we produced a score sheet (Appendix 1, Table S2) and methodological criteria based on the criteria of Zuo and colleagues.8 The literature search, study evaluation and study selection were conducted independently by 2 investigators (J.Y.G. and Z.Z.Q.). Any discrepancies were resolved by a third investigator (Y.W.) for a final decision. The current study was conducted with reference to the Meta-analysis of Observational Studies in Epidemiology guidelines for the meta-analyses of observational studies.34 This checklist was adopted to rate only the completeness of the reported methods and results without criticizing the work itself or the investigators.
Data analysis
We conducted meta-analyses of regional homogeneity differences between patients and healthy controls separately for schizophrenia and bipolar disorder using a seed-based d-mapping (SDM) software package (version 5.15 for Windows) and a standard process (www.sdmproject.com). The SDM approach uses effect sizes to combine reported peak coordinates that are extracted from databases with statistical parametric maps, and it recreates original maps of the effect size of regional homogeneity difference between patients and healthy controls. We performed the analysis as described in the SDM tutorial and related publications and used MRIcron software (www.mricro.com/mricron/) to visualize the SDM maps.
The SDM approach is described here. Briefly, we first extracted peak coordinates and effect sizes (e.g., t values) for differences in regional homogeneity between patients and healthy controls from each data set. We then recreated a standard Montreal Neurological Institute map of the regional homogeneity differences separately for each data set using an anisotropic Gaussian kernel. Finally, we generated the mean map by voxel-wise calculation of the random-effects mean of the data set maps, weighted by sample size, intra-data-set variability and between-data-set heterogeneity. We used the default SDM kernel size and thresholds (full width at half maximum [FWHM] = 20 mm; p = 0.005, uncorrected for false discovery rate; peak height Z = 1; cluster extent = 10 voxels)35–40 as used in many previous studies because they have been validated to optimize sensitivity and specificity and to produce a desirable balance between type I and II error rates.41 This FWHM kernel is intended to assign indicators of proximity to reported coordinates but not to smooth any image that is different in nature.
We also investigated the overlap of increases or decreases in regional homogeneity between schizophrenia and bipolar disorder by overlapping the thresholded meta-analytic results maps.
We repeated the above meta-analyses in 2 subgroups (unmedicated patients with first-episode schizophrenia and patients with bipolar disorder and depression). We did not conduct the same analyses in other subgroups (i.e., bipolar disorder I and II; euthymic and manic states of bipolar disorder; unmedicated patients with bipolar disorder) because of a limited number of studies (n < 10).
To confirm our results, we conducted validation across versions using the latest version of SDM (SDM-PSI [permutation of subject images], version 6.21) to reanalyze the data.
Jackknife sensitivity analysis
Following preprocessing of the data, we performed whole-brain voxel-based jackknife sensitivity analysis to test the robustness of the findings by iteratively repeating the same analysis, excluding 1 data set at a time.35 This analysis was to establish the extent to which the results could be replicated. If a brain region remained significant in all or most (> 90%) of the study combinations, we considered the finding to be highly replicable.42,43
Analysis of heterogeneity and publication bias
We conducted a heterogeneity analysis using a random-effects model with Q statistics to explore unexplained between-study variability in the results. We obtained heterogeneous brain regions using the default SDM kernel size and thresholds described above.35–40
We also performed Egger tests using Stata/SE 12.0 for Windows (Stata Corp. LP) to assess possible publication bias by extracting values from significant relevant peaks between patients and healthy controls.35 A p value of less than 0.05 was considered significant.
Meta-regression analyses
We carried out meta-regression analyses to examine the effects of clinical variables that could influence the detected between-group differences (e.g., illness duration and total score on the Positive and Negative Syndrome Scale for schizophrenia; illness duration and scores on the Hamilton Depression Rating Scale and Young Mania Rating Scale for bipolar disorder). The results were weighted by the square root of the sample size. To minimize the reporting of spurious relationship, we selected a more conservative threshold of p = 0.0005 as used in previous studies,35 requiring alterations to be detected both in the slope and in 1 of the extremes of the regressor, and discarding findings in regions other than those detected in the main analyses.
Meta-analysis of voxel-based morphometry changes to schizophrenia and bipolar disorder
We used whole-brain voxel-based morphometry meta-analysis of structural imaging studies to determine the structural substrates of altered regional homogeneity in patients with schizophrenia or bipolar disorder. Consistent with the meta-analysis of regional homogeneity studies, we performed a similar procedure to select studies related to voxel-based morphometry analysis. We extracted peak coordinates with decreased and increased volumes for each study separately. We also performed voxel-based morphometry meta-analysis using the multi-level kernel density analysis algorithm described above.
Multimodal overlap of altered regional homogeneity and altered grey matter volume in schizophrenia or bipolar disorder
We investigated the overlap of increased or decreased regional homogeneity in patients with schizophrenia or bipolar disorder with grey matter volume increases or decreases by overlapping the thresholded meta-analysis results maps above.
Results
Included studies and sample characteristics
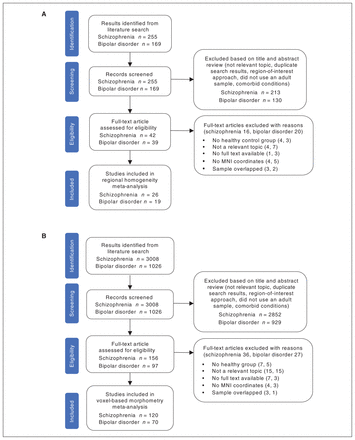
A flow chart describing the identification and exclusion of studies is shown in Figure 1.
Flow chart of study selection for (A) regional homogeneity and (B) voxel-based morphometry. MNI = Montreal Neurological Institute.
For the regional homogeneity meta-analysis, we selected 29 studies from 26 publications, comprising 1325 patients with schizophrenia and 1178 healthy controls; we also selected 21 studies from 19 publications, comprising 708 patients with bipolar disorder and 1000 healthy controls. We observed no significant differences between patients with schizophrenia or bipolar disorder and healthy controls with respect to age (schizophrenia: 95% confidence interval [CI] −3.621 to 1.618, t = −0.764, p = 0.45; bipolar disorder: 95% CI −2.732 to 3.128, t = 0.137, p = 0.89) or sex distribution (schizophrenia: χ2 = 2.166, p = 0.14; bipolar disorder: χ2 = 0.588, p = 0.44).
For the voxel-based morphometry meta-analysis, we selected 141 studies with 120 publications, comprising 5351 patients with schizophrenia and 5591 healthy controls; we also included 76 studies with 70 publications, comprising 2583 patients with bipolar disorder and 3081 healthy controls. We observed no significant differences between patients with schizophrenia or bipolar disorder and healthy controls with respect to age (schizophrenia: 95% CI −1.123 to 2.655, t = 0.799, p = 0.43; bipolar disorder: 95% CI 0.768 to 2.069, t = 0.819, p = 0.13) or sex distribution (schizophrenia: χ2 = 0.231, p = 0.63; bipolar disorder: χ2 = 2.058, p = 0.15).
The mean quality scores of the included studies for schizophrenia and bipolar disorder were 9.5 (range 8.0–10.0) and 9.4 (range 8.0–10.0), respectively, showing that the studies were of high and similar quality across disorders. Detailed demographic, clinical and imaging characteristics, as well as quality scores for the included studies, are summarized in Appendix 1, Tables S1, S2 and S3A–D.
Differences in regional homogeneity: main meta-analysis
Schizophrenia versus healthy controls
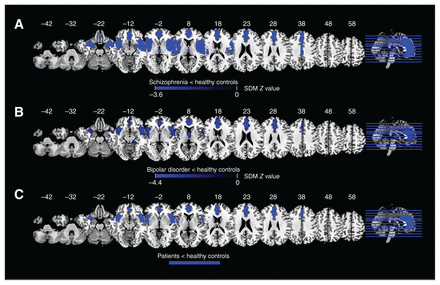
Compared to healthy controls, patients with schizophrenia displayed increased regional homogeneity in the right inferior frontal gyrus (IFG) extending to the right insula, right striatum, right putamen and right superior temporal gyrus (STG); the right superior frontal gyrus (SFG) extending to the bilateral anterior cingulate cortex (ACC) and supplementary motor area (SMA); and the left caudate nucleus extending to the bilateral striatum, left SFG and right SMA (Figure 2A and Table 1).
Results of the meta-analyses for regional homogeneity: (A) difference between patients with schizophrenia and healthy controls; (B) difference between patients with bipolar disorder and healthy controls; (C) overlap between patients with schizophrenia and bipolar disorder. Areas with decreased regional homogeneity are displayed in blue, and areas with increased regional homogeneity are displayed in red. The colour bar indicates the maximum and minimum SDM Z values. SDM = seed-based d-mapping.
Differences in regional homogeneity between patients with schizophrenia or bipolar disorder and healthy controls — results of the meta-analysis
Patients with schizophrenia also displayed decreased regional homogeneity in the left Heschl gyrus extending to the left insula, left STG and left postcentral gyrus; the right middle temporal gyrus (MTG) extending to the right inferior occipital gyrus and the right inferior temporal gyrus (ITG); the right precentral gyrus; the right cuneus cortex extending to the right superior occipital gyrus; the left postcentral gyrus; the left inferior occipital gyrus; and the right paracentral lobule.
These areas did not show significant between-study heterogeneity. Egger tests of publication bias were non-significant. Jackknife sensitivity analysis revealed that these results were robust and replicable in all data sets.
The results for differences in regional homogeneity between unmedicated patients with first-episode schizophrenia and healthy controls are shown in Appendix 1, Figure S1A, and Table S4. The main results remained largely unchanged.
Bipolar disorder versus healthy controls
Compared to healthy controls, patients with bipolar disorder displayed increased regional homogeneity in the left IFG extending to the left middle frontal gyrus, the right SFG extending to the right middle frontal gyrus and right SMA, the right gyrus rectus, the right striatum, and the left inferior occipital gyrus (Figure 2B and Table 1).
Patients with bipolar disorder also displayed decreased regional homogeneity in the left Heschl gyrus extending to the left insula, left STG, left putamen, left postcentral gyrus and left striatum; the right ITG extending to the right inferior and middle occipital gyrus; the right thalamus; and the left postcentral gyrus.
These areas did not show significant between-study heterogeneity. Egger tests of publication bias were non-significant except for the left inferior occipital gyrus, the left Heschl gyrus and left postcentral gyrus (p < 0.05). Jackknife sensitivity analysis revealed that in patients with bipolar disorder, these results were robust and replicable in all data sets.
The results for differences in regional homogeneity between patients with bipolar disorder and depression and healthy controls are shown in Appendix 1, Figure S1B, and Table S5. The main results remained largely unchanged.
Overlap of schizophrenia and bipolar disorder
Overlap analysis revealed that, compared to healthy controls, patients with schizophrenia and patients with bipolar disorder showed increased regional homogeneity in the bilateral SFG (mainly in the medial prefrontal cortex [mPFC]), and decreased regional homogeneity in the left insula extending to the STG, right STG and ITG (Figure 2C).
Differences in voxel-based morphometry: main meta-analysis
Schizophrenia versus healthy controls
Compared to healthy controls, patients with schizophrenia displayed decreased grey matter volume in the right STG extending to the right insula, right STG, right MTG, right striatum, right postcentral gyrus and right precentral gyrus; the left IFG extending to the left insula, left STG, left MTG, left amygdala, left postcentral gyrus and left precentral gyrus; the bilateral ACC extending to the bilateral SFG and bilateral median cingulate cortex; the right thalamus; and the left cerebellum (Figure 3A and Table 2).
Results of the meta-analyses results for voxel-based morphometry: (A) difference between patients with schizophrenia and healthy controls; (B) difference between patients with bipolar disorder and healthy controls; (C) overlap between patients with schizophrenia and bipolar disorder. Areas with decreased voxel-based morphometry are displayed in blue, and areas with increased voxel-based morphometry are displayed in red. The colour bar indicates the maximum and minimum SDM Z values. SDM = seed-based d-mapping.
Differences in voxel-based morphometry between patients with schizophrenia or bipolar disorder and healthy controls — results of the meta-analysis
Bipolar disorder versus healthy controls
Compared to healthy controls, patients with bipolar disorder displayed decreased grey matter volume in the right ACC extending to the left ACC, bilateral SFG and bilateral median cingulate cortex; the right insula extending to the right STG, right IFG and right MTG; the left IFG extending to the left insula and left STG; the right thalamus; and the right fusiform gyrus (Figure 3B and Table 2).
Overlap of schizophrenia and bipolar disorder
Overlap analysis revealed that, compared to healthy controls, patients with schizophrenia and patients with bipolar disorder showed decreased grey matter volume in the bilateral medial prefrontal cortex and ACC extending to the midcingulate cortex; the bilateral IFG; and the bilateral insula extending to the STG (Figure 3C).
Overlap of altered regional homogeneity and altered grey matter volume in schizophrenia and bipolar disorder
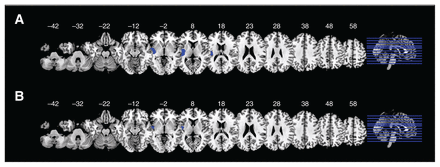
Patients with schizophrenia displayed decreased regional homogeneity and decreased voxel-based morphometry in the left insula and left STG. Patients with bipolar disorder displayed decreased regional homogeneity and decreased voxel-based morphometry in the left insula (Figure 4).
Overlap of decreases in regional homogeneity and voxel-based morphometry in (A) patients with schizophrenia and (B) patients with bipolar disorder.
Meta-regression analyses
In patients with schizophrenia, meta-regression analysis indicated that longer illness duration was correlated with greater increases in regional homogeneity of the right insula, left SMA, right MFG and right caudate nucleus. In patients with bipolar disorder, longer illness duration was correlated with greater decreases in regional homogeneity of the right MFG; a higher score on the Hamilton Depression Rating Scale was correlated with greater decreases in regional homogeneity of the right thalamus; and a higher score on the Young Mania Rating Scale was correlated with greater increases in regional homogeneity of the bilateral IFG and greater decreases in regional homogeneity of the left Heschl gyrus (Table 3).
Regional homogeneity for schizophrenia and bipolar disorder — results of the meta-regression analyses
Validation across versions
In the regional homogeneity meta-analysis, no significant cluster survived when we reported clusters at a more liberal threshold (family-wise error–corrected p < 0.05) with clusters of at least 10 voxels using the latest version of SDM-PSI 6.21 to reanalyze the data.
In the voxel-based morphometry analysis, patients with schizophrenia displayed decreased grey matter volume in the bilateral STG, left ACC and right median cingulate gyri compared to healthy controls. Patients with bipolar disorder displayed decreased grey matter volume in the right insula compared with healthy controls (Appendix 1, Table S8, Figure S3).
Discussion
As far as we know, this is the first quantitative meta-analysis to compare whole-brain differences in regional homogeneity and grey matter volume from resting-state fMRI and voxel-based morphometry studies in patients with schizophrenia and bipolar disorder. We compared results to identify both overlapping and distinctive patterns. We found that patients with schizophrenia and bipolar disorder shared decreased regional homogeneity in the left insula extending to the STG, right STG and ITG, and increased regional homogeneity in the frontal cortex, mainly in the medial prefrontal cortex. They also showed decreased grey matter volume in the bilateral medial prefrontal cortex and ACC, bilateral IFG and bilateral insula extending to the STG, possibly implying that localized change in connectivity overlaps with structural deficits. Moreover, patients with schizophrenia showed decreased regional homogeneity in the primary sensory cortex (visual and auditory cortices) and SMA, and decreased grey matter volume in the cerebellum, suggesting more widespread functional and structural alterations in schizophrenia.
Multimodal overlap analyses showed decreased regional homogeneity and voxel-based morphometry in the left insula and STG in patients with schizophrenia, and decreased regional homogeneity and voxel-based morphometry in the left insula in patients with bipolar disorder. Although the literature search revealed some publication bias, we verified the main results to be highly replicable and stable using jackknife sensitivity analysis.
The insula, a part of the paralimbic structure, is considered a gateway between the somatosensory cortex and limbic structures.44 It is believed to be involved in diverse functions, including perception,45 motor control,46 cognitive function,47 self-awareness48 and emotion.49 Our meta-analysis found consistent regions of decreased regional homogeneity and grey matter volume in the insula in patients with schizophrenia and bipolar disorder, suggesting impaired regional neural activity and volume in the insula in both disorders. Furthermore, patients with schizophrenia displayed more reduced grey matter volume in the insula than patients with bipolar disorder. Several studies have found reduced cerebral blood flow,50,51 metabolism,52–54 functional activation55–57 and connectivity58,59 in the insula in patients with schizophrenia and bipolar disorder. Previous meta-analysis of voxel-based morphometry studies have identified grey matter volume reductions in the bilateral insula in patients with schizophrenia31,60 and bipolar disorder.44,61,62 Similarly, 2 meta-analyses of voxel-based morphometry studies reported decreased insula volumes in drug-naive patients with first-episode schizophrenia63 and their healthy relatives,64 indicating that the insula deficit is a potential marker of risk. Furthermore, proteomic analysis of the insula in patients with schizophrenia found altered expression of multiple proteins that participate in synaptic function and neuronal morphogenesis.65
A recent task-based meta-analysis of neuroimaging studies comparing patients with psychiatric disorders (including schizophrenia and bipolar disorder) with healthy controls found that disrupted mid-insular activation may represent a neural marker of psychopathology and a putative target for novel interventions.66,67 Meta-regression analysis demonstrated that longer illness duration was correlated with greater increases in regional homogeneity of the right insula in schizophrenia. These structural and functional deficits of the insula in schizophrenia and bipolar disorder during information processing suggest disturbance to the system that effects changes between contextually relevant functional brain states.47,68–73 Taken together, functional and structural impairment of the insula may be a shared key neurobiological feature of schizophrenia and bipolar disorder.
The frontostriatal circuits connect the prefrontal cortices with the striatum, which subserves emotional and cognitive function. The current meta-analysis found increased regional homogeneity in the bilateral frontal cortex (including the superior, middle and inferior frontal gyrus) and the striatum, and decreased grey matter volume in the mPFC in patients with schizophrenia and bipolar disorder. These differences in regional homogeneity and grey matter volume were less extensive in patients with bipolar disorder than in patients with schizophrenia, but they overlapped substantially. In particular, our overlap analysis revealed that patients with schizophrenia and patients with bipolar disorder had increased regional homogeneity and decreased grey matter volume in the bilateral mPFC, suggesting a consistent functional and structural altered pattern across disorders.
Many brain imaging studies have confirmed that activity in the frontal cortex is associated with many aspects of directed effort, such as the ability to represent the thoughts, feelings and actions of self and others across time, known as mentalizing.74 Functional alterations of the frontal lobe have been among the most consistent findings in schizophrenia75,76 and bipolar disorder,76 although they have not generally been localized to the mPFC.77 A task-based fMRI study using the Montreal Imaging Stress Task found increased amygdala–prefrontal cortex (PFC) functional connectivity during stress processing in bipolar disorder; greater increases in amygdala–PFC functional connectivity was associated with less frequent cannabis use, and prospectively with shorter duration and lower severity of depression symptoms at follow-up.78 Meta-analysis of voxel-based morphometry studies reported grey matter reductions in the frontal cortex (including the mPFC) in patients with schizophrenia63,79 and bipolar disorder.62,80,81 Previous diffusion tensor imaging studies found reduced frontostriatal structural connectivity in patients with schizophrenia,79,82,83 which was associated with cognitive impairment.76,84 It is worth noting that the striatum and the PFC are intermodulated via frontostriatal circuits modulated by dopamine.79 A meta-analysis of studies using positron emission tomography and single photon emission computed tomography showed increased striatal dopamine synthesis and release in patients with schizophrenia.85
Meta-regression analysis indicated that longer illness duration was correlated with greater increases in regional homogeneity of the right MFG in patients with schizophrenia and greater decreases in regional homogeneity of the right MFG in patients with bipolar disorder; a higher score on the Young Mania Rating Scale was correlated with greater increases in regional homogeneity of the bilateral IFG in patients with bipolar disorder. Volume reductions in prefrontal areas, increased density in subcortical areas, and morphologic alterations in oligodendroglia have all been related to schizophrenia.86–89 These findings of increased functional connectivity in the frontal cortex and striatum may represent a compensatory response to structural deficits in both disorders, providing additional evidence for the involvement of frontostriatal dysconnectivity in the pathophysiology of schizophrenia and bipolar disorder.
Patients with schizophrenia showed decreased regional homogeneity in the primary sensory cortex (visual and auditory cortices) and sensorimotor cortex, indicating more widespread intrinsic activity alterations in schizophrenia. Furthermore, overlap analyses demonstrated decreased regional homogeneity and voxel-based morphometry in the left STG and left insula in patients with schizophrenia. Functional and structural alterations in the superior temporal90–92 and occipital cortices93,94 may be correlated with the clinical characteristics of auditory and visual hallucinations in patients with schizophrenia.95,96 We did not find such differences in bipolar disorder. Furthermore, in the present study, patients with schizophrenia displayed decreased regional homogeneity and grey matter volume in the precentral gyrus and postcentral gyrus, suggesting that functional and structural alteration in these sensorimotor regions may aid in distinguishing the 2 disorders.
Our recent meta-analysis also reported decreased amplitude of low-frequency fluctuation in the bilateral postcentral gyrus in patients with schizophrenia, particularly chronic schizophrenia,75,76 suggesting much more widespread damage with the progression of disease. Previous studies found that an impaired sensorimotor system was related to psychomotor alterations such as psychomotor retardation, dyskinetic syndrome and psychomotor agitation in patients with schizophrenia.76,97–99 Using a novel fMRI task during simulation of sensorimotor experiences, 1 study found that simulation elicited specific neural representations in the sensorimotor cortices, and the strength of these representations might be linked to social function.100 Recent findings showed γ-aminobutyric acid (GABA) cell reductions in the primary sensory cortex and association cortex in patients with depression, consistent with previous demonstrations of comparable GABA reductions in the prefrontal association and occipital visual sensory areas.101 Previous studies of the auditory cortex in patients with schizophrenia found subtle thinning of the planum temporale, fewer glia, reduced neuron size and unchanged neuron density.102,103 These results suggest irreversible neuronal loss in patients with schizophrenia, which may be a consequence of disease associated with neurotoxicity, or as a pre-existing condition that contributes to vulnerability to schizophrenia. These findings of disrupted intrinsic regional activity in the primary sensory motor areas may contribute to the typical symptoms of hallucination and psychomotor disturbances in schizophrenia. In combination with the findings of increased regional homogeneity in the frontal cortex, they might reflect the broken balance between advanced cognitive and primary sensory motor function in patients with schizophrenia.
Limitations
Our study had several limitations. First, the selected studies included medicated and unmedicated patients, making it difficult to exclude the possibility that the observed brain changes occurred as a consequence of illness or its treatment. Second, because of a limited number of studies, we did not further investigate subgroups of bipolar disorder, such as bipolar disorder I and II, or the remission and manic stages of the disorder. Third, our meta-analysis was based on coordinates from published studies rather than on raw data, limiting its accuracy.104 Fourth, we found substantial heterogeneity in our results for some regions with altered regional homogeneity, a finding that may be attributable to the clinical diversity of the participants and the methodological and statistical diversity of the studies, partially limiting the interpretability and generalizability of the results. Finally, we used an uncorrected threshold of p < 0.005 to report our results. When using a more conservative statistical threshold (p < 0.05, family-wise error–corrected), the meta-analyses yielded no significant results for regional homogeneity. Possible reasons for this may be the limited number of studies in the regional homogeneity meta-analysis and their heterogeneity. Future studies are needed to validate our results.105
Conclusion
The current meta-analysis demonstrates that schizophrenia and bipolar disorder share a common pattern of regional functional and structural alterations in the insula and frontal cortex (particularly the mPFC). Moreover, patients with schizophrenia showed more widespread functional and structural impairment, most prominently in the primary sensory motor areas. These results expand on a growing literature that explores resting-state activity and structural change in patients with schizophrenia and bipolar disorder, providing useful insights into the underlying pathophysiology of brain dysfunction and structural impairment in schizophrenia and bipolar disorder, and helping to develop more targeted and efficacious treatment and intervention strategies.
Footnotes
↵* These authors contributed equally to this work.
Funding: The study was supported by grants from the National Natural Science Foundation of China (81801685, 81971597 and 82102003); the Project in Basic Research and Applied Basic Research in General Colleges and Universities of Guangdong, China (2018KZDXM009); and the National Key Research and Development Project (2020YFC2005700). The funding organizations played no further role in study design, data collection, analysis and interpretation or writing.
Competing interests: None declared.
Contributors: Y. Wang and L. Huang designed the study. Z. Qi, T. Su and S. Fu acquired the data, which Z. Qi, J. Wang, J. Gong and Y. Wang analyzed. Z. Qi and J. Wang wrote the article, which J. Gong, T. Su, S. Fu, L. Huang and Y. Wang reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
- Received July 8, 2021.
- Revision received October 12, 2021.
- Accepted November 16, 2021.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/