Abstract
Background: Despite a large body of schizophrenia research, we still have no reliable predictors to guide treatment from illness onset. The present study aimed to identify baseline clinical or neurobiological factors — including peripheral brain-derived neurotrophic factor (BDNF) levels and amygdala or hippocampal relative volumes — that could predict negative symptomatology and persistent negative symptoms in first-episode psychosis after 1 year of follow-up.
Methods: We recruited 50 drug-naive patients with first-episode psychosis and 50 age- and sex-matched healthy controls to study brain volumes. We performed univariate and multiple and logistic regression analyses to determine the association between baseline clinical and neurobiological variables, score on the PANSS negative subscale and persistent negative symptoms after 1 year of follow-up.
Results: Low baseline serum BDNF levels (p = 0.011), decreased left amygdala relative volume (p = 0.001) and more severe negative symptomatology (p = 0.021) predicted the severity of negative symptoms at 1 year, as measured by the PANSS negative subscale. Low baseline serum BDNF levels (p = 0.012) and decreased left amygdala relative volume (p = 0.010) predicted persistent negative symptoms at 1 year.
Limitations: We were unable to assess negative symptoms and their dimensions with next-generation scales, which were not available when the study was initiated.
Conclusion: This study shows that a set of variables at baseline, including low BDNF levels, smaller left amygdala relative volume and score on the PANSS negative subscale are significant predictors of outcomes in first-episode psychosis. These findings might offer an initial step for tailoring treatments in first-episode psychosis.
Introduction
Schizophrenia is a chronic, lifelong, disabling disorder that develops via complex, heterogeneous, multifactorial (genetic, developmental and environmental) pathways.1 It is 1 of the top 15 leading causes of disability worldwide,2 impairing critical areas of everyday functioning.3 Although early interventions may improve treatment outcomes,4 there is a notable lack of reliable predictors to help guide an objective, data-driven personalized approach to treatment after illness onset.5
Given the complexity of schizophrenia, it is essential to identify unambiguous and distinctive predictors of outcomes, 6 such as negative symptoms.7 Negative symptoms are among the most important determinants of functioning8,9 in schizophrenia. They can be present at any stage of the disorder, even in the early stages, with prevalence in up to 50% of patients.10 Longitudinal studies in early stages have found that when these symptoms are present at baseline, they tend to be persistent, although severity may vary over time. Therefore, it would be highly valuable to identify — at or near disease onset — those patients who are most likely to develop severe negative symptoms; this would facilitate the early implementation of personalized interventions.11
There are different approaches to measuring negative symptoms. The most common approach is to rate all negative symptoms without considering the possible cause (e.g., positive symptoms, adverse effects, social deprivation or others); PANSS is one of the most widely used scales for this purpose. Another approach is to restrict negative symptoms to those considered primary or deficit symptoms12,13 after ruling out secondary causes. An alternative approach to studying negative symptoms is to use persistent negative symptoms; these may include secondary negative symptoms, but only those that have failed to respond to the usual treatments and are evident for more than 6 months. Persistent negative symptoms are also highly relevant because they lead to functional impairment and represent an unmet therapeutic need.14
Several clinical factors have been linked to negative symptoms in general. For example, more severe negative symptoms have been observed in males,15,16 in patients who developed schizophrenia at a younger age17,18 and in those with a longer duration of untreated psychosis.19,20 However, the association between negative symptoms and these clinical factors has not been consistent in the studies to date.15,19,21 The influence of cannabis use on negative symptomatology is poorly understood, but a recent meta-analysis showed that recently abstinent patients showed negative symptoms that were less severe than nonusers.22 In addition to these clinical markers, a considerable amount of research has been conducted to identify neurobiological factors that could predict outcomes and help to select optimal treatment strategies.5
Morphological and functional brain alterations have been widely reported in schizophrenia.23 Structural and functional changes in the limbic system and medial temporal lobe are central to the symptomatology associated with psychosis, 24 and smaller relative hippocampal volumes are commonly observed in patients with first-episode psychosis and schizophrenia.25 Furthermore, these hippocampal abnormalities are associated with the emergence of negative symptoms and persistent negative symptoms in patients with schizophrenia and first-episode psychosis.10,26 Smaller amygdala volumes have also been reported in patients with schizophrenia and first-episode psychosis compared to controls.27,28 In addition, volume alterations in the amygdala have been associated with both negative symptoms and persistent negative symptoms in schizophrenia and in first-episode psychosis during follow-up.10,26
Brain-derived neurotrophic factor (BDNF) has been implicated in the pathophysiology of schizophrenia, and it has been shown to play a crucial role in neuronal development, transmission, regulation and synaptic plasticity.29 Data from systematic reviews and meta-analyses show that patients with schizophrenia30 and first-episode psychosis31 have lower peripheral BDNF levels than healthy controls. Cross-sectional studies have found an association between peripheral BDNF levels and the clinical features of schizophrenia, such as the severity of negative symptoms.32 These data suggest that peripheral BDNF levels could be a neurobiological predictor of psychosis and psychosis phenotypes. However, more longitudinal studies are needed in first-episode psychosis to better determine the association between peripheral BDNF levels and different outcome parameters, including negative symptoms.
In summary, there is a clear need to identify predictors of outcomes in schizophrenia and guide individualized treatment strategies from illness onset. Negative symptoms are distinctive features of schizophrenia and critical determinants of outcomes. Although various sociodemographic, clinical and neurobiological factors have been associated with negative symptoms, few studies have evaluated the predictive value of these factors longitudinally starting from disease onset, and even fewer have considered the sources of secondary negative symptoms or persistent negative symptoms. Finally, to our knowledge, no studies have assessed all 3 factors (clinical, neuroimaging and biochemical) together.
The primary objective of the present study was to determine whether baseline clinical and neurobiological factors — including duration of untreated psychosis, clinical symptoms, cannabis use, peripheral BDNF levels and amygdala or hippocampal relative volumes — could predict negative symptomatology and persistent negative symptoms at 1 year of follow-up in patients with first-episode psychosis. A second aim was to measure and compare hippocampal and amygdalar volumes in patients with first-episode psychosis and healthy controls to determine significant differences.
We hypothesized that this combination of clinical and neurobiological parameters would predict negative symptoms and persistent negative symptoms at 1-year follow-up. We also hypothesized that patients with first-episode psychosis would have smaller hippocampal and amygdalar relative volumes than healthy controls.
Methods
Study population
Fifty consecutive, drug-naive patients with first-episode psychosis who were treated between April 2013 and July 2017 at the Estudi i Tractament de Primers Episodis Psicotics (ETEP) Program at Hospital del Mar (Barcelona, Spain) were included in this study. The ETEP program is a specialized early intervention service for people aged 18 to 35 who experience first-episode psychosis. It provides multimodal intervention, including extensive assessment and intensive medical and psychosocial treatment (for more details, see Bergé and colleagues33).
For the present study, inclusion criteria for patients were as follows: age 18 to 35 years; fulfillment of DSM-IV-TR criteria for brief psychotic disorder, schizophreniform disorder, schizophrenia with less than 1 year of symptoms or unspecified psychosis; no previous history of severe neurologic medical conditions or severe traumatic brain injury; presumed IQ above 80 based on clinical records (evidence from previous IQ assessments or suggested by the patient’s educational or employment level); and no substance abuse or dependence disorders, except for cannabis or nicotine use. All patients were antipsychotic-and antidepressant-naive at inclusion. Treatment with benzodiazepines was allowed. We excluded affective psychosis to homogenize the sample, because negative symptoms in affective psychosis can differ in terms of whether they are primary or secondary and persistent or transient34 (see Appendix 1, Figure S1 and Table S1, available at www.jpn.ca/lookup/doi/10.1503/jpn.210138/tab-related-content, for detailed information on patients excluded from the neuroimaging study).
We used 50 age- and sex-matched healthy volunteers as a control group for anatomic measurements. All healthy controls underwent a complete medical interview. Those with relevant medical or neurologic disorders, substance abuse disorders or psychiatric illnesses were excluded from the study. None of the healthy controls was undergoing medical treatment at study inclusion. Given the characteristics of the study design, we used no statistical methods to predetermine sample sizes.
The local ethics committee approved this study, and all participants provided written informed consent.
Clinical assessment and demographic data
All patients underwent a comprehensive assessment at baseline and 1-year follow-up, performed by 2 experienced psychiatrists (A.M., D.B.). The assessment included sociodemographic variables; the Structured Clinical Interview for DSM-IV-TR Axis I disorders for diagnosis; an assessment of substance use, including tobacco (cigarettes per day) and cannabis use (joints per week, after dichotomization between user/nonuser); the Positive and Negative Syndrome Scale (PANSS) for symptoms related to psychosis; 35 the Global Assessment of Functioning (GAF)36 for functionality; and the Calgary Depression Scale for Schizophrenia (CDSS) for depressive symptoms.37 We collected patients’ antipsychotic dose at 1-year follow-up from their medical records. We converted doses of antipsychotic medications to chlorpromazine equivalents (mg/d) to facilitate comparisons.38
To minimize the effect of secondary negative symptom sources, we excluded all patients who scored 6 or higher on the CDSS at baseline or at follow-up.39 We also included antipsychotic treatments at follow-up in the analyses.
We also assessed for the presence of persistent negative symptoms, defined as 1 or more negative symptoms of moderate or higher severity (PANSS negative subscale > 3), not confounded by depression (CDSS < 6) or parkinsonism at baseline and after 1 year of treatment.39
Collection of blood samples and determination of serum BDNF levels in patients at baseline
We obtained baseline fasting blood samples upon patients’ arrival at the health centre before administering any medication (except for benzodiazepines). All blood samples were obtained in the morning (between 8 am and 12 pm) to avoid circadian fluctuations in BDNF levels, which have been reported to occur in men but not in women.40 Blood samples were collected in glass K3–EDTA blood-drawing tubes for whole blood. Serum was isolated by centrifugation at 300 × g for 15 minutes, and then removed and stored at −80°C until analysis.
BDNF levels were measured using a sandwich enzyme-linked immunoabsorbent assay kit according to the manufacturer’s instructions (ChemiKine; Chemicon). Absorbencies were determined using the Wallac Victor 2 microplate reader, with the wavelength set at 450 nm. BDNF concentrations were detected according to the standard curve, which was constructed from duplicate samples containing appropriate concentrations. All samples were analyzed in duplicate. The calculated overall intra- and interassay variation coefficients were 3.7% and 8.5%, respectively. The detection limit of the BDNF assay was 15 pg/mL.
Image acquisition and processing at baseline
Brain imaging was performed with a Philips Achieva 3.0 T MRI scanner (Philips Healthcare) equipped with an 8-channel phased-array head coil. The imaging protocol involved the acquisition of high-resolution anatomic 3-dimensional images based on a T1-weighted fast spoiled gradient inversion recovery prepared sequence with the following parameters: repetition time 8.2 ms; echo time 3.8 ms; flip angle 8°; field of view 24 cm; matrix 256 × 256 pixels; in-plane resolution 0.94 × 0.94 mm2; and slice thickness 1 mm.
Anatomic images were visually inspected before analysis by a trained operator to detect any motion effect. No gross brain abnormalities were identified. The quality of the raw T1-weighted MRI scans was quantitatively assessed using the automated weighted image quality rating (a measure of general image quality that combines the parameters of noise, inhomogeneities and spatial resolution) in the CAT12 toolbox (http://dbm.neuro.uni-jena.de/cat/); a score of more than 60% is considered sufficient quality for inclusion in subsequent analyses. All scans passed this threshold.
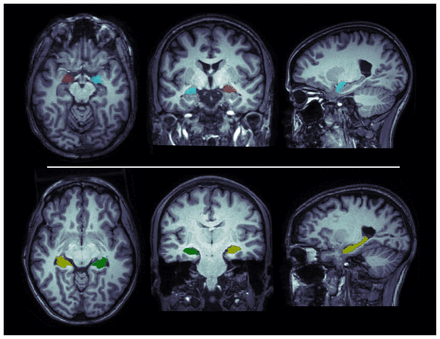
We performed volumetric segmentation using the fully automated and validated segmentation software package FreeSurfer v6.0 (http://surfer.nmr.mgh.harvard.edu) with the default “recon-all” stream, as described in previous studies.41–44 Briefly, important preprocessing steps included removal of non-brain tissue, intensity normalization, automated Talairach transformation and segmentation of the subcortical white matter and deep grey matter volumetric structures.45 Segmentations of the hippocampus and amygdala (left and right hemisphere) were visually inspected for accuracy by overlaying the segmentation label of each structure on the individual T1-weighted brain scan. Specifically, we used the imaging quality control protocol for subcortical segmentations developed by the ENIGMA consortium (http://enigma.ini.usc.edu/protocols/imaging-protocols/) to generate a standard set of images from every participant’s brain scan, displaying boundaries of segmented structures on a series of slices in the axial, coronal and sagittal planes (Figure 1) for visual inspection of segmentation inaccuracies. For each participant, we extracted estimated hippocampal and amygdalar volumes (left and right hemisphere), as well as estimated total intracranial volume, from the aseg.stats output files in FreeSurfer (https://surfer.nmr.mgh.harvard.edu/fswiki/asegstats2table). We used group-level means, standard deviations and histogram plots to identify statistical outliers (i.e., if their volume was measured to be greater than 2.698 standard deviations [SDs] from the global mean) and to confirm the normal distribution of volumetric data. One control participant was identified as an outlier for the estimate of right amygdala volume. In this case, we visually reinspected the images using Freeview (v2.0) in FreeSurfer to confirm segmentation accuracy, and the data were retained in the analysis.
Illustrations of the amygdala (top row) and the hippocampus (bottom row) as segmented by FreeSurfer in a control participant. Segmentations are overlaid on the participant’s anatomic MRI.
We calculated the relative volume ratio for the anatomic regions of interest as the volume in native space (mL) divided by the total intracranial volume (L). We performed MRI assessments when patients were able to stand still (i.e., they did not have high psychomotor agitation or disorganized behaviour and could tolerate the MRI procedure). The scan was taken within 2 weeks of the initial assessment.
Statistical analysis
We assessed data distribution normality using the Kolmogorov–Smirnov test. Only age, duration of untreated psychosis and tobacco use were non-Gaussian variables. We performed univariate analyses to evaluate differences between the patients who completed the entire follow-up and those who did not. We also performed univariate analyses to assess between-group differences (patients and healthy controls), and Bonferroni correction on brain-volume comparisons.
To determine predictors of negative symptoms at 1-year follow-up, we first conducted univariate analyses that included sociodemographic and baseline clinical and neurobiological variables that were potentially associated with scores on the PANSS negative subscale at 1-year follow-up: sex, age, duration of untreated psychosis, baseline CDSS score, PANSS positive subscale score, benzodiazepine treatment, left and right amygdala relative volumes, left and right hippocampal relative volumes and serum BDNF levels. We also accounted for antipsychotic treatment at follow-up as a possible confounding factor.
Next, we developed a linear regression model using the PANSS negative subscale at 1-year follow-up as the dependent variable. Independent variables were all baseline variables (age, sex, cannabis use, tobacco use, duration of untreated psychosis, PANSS negative subscale, PANSS positive subscale, CDSS, GAF, BDNF, left and right amygdala relative volume, left and right hippocampus relative volume, and benzodiazepine treatment) and chlorpromazine equivalents at 1 year follow-up. We also performed an exploratory analysis to assess predictors of the PANSS negative subscale items using the same methodology.
To determine predictors of persistent negative symptoms at 1-year follow-up, we used the same methodology, except that we used a logistic regression model with persistent negative symptoms (yes/no) as the dependent variable.
All statistical analyses were performed in SPSS Statistics for Windows, version 20 (IBM Corp.); p values ≤ 0.05 were considered statistically significant.
Results
Characteristics of patients and healthy controls
A total of 50 patients were initially included in the study. None of the patients scored 6 or higher on the CDSS at baseline or at follow-up. More than half (56%) of the patients were male. The median age was 26 years (interquartile range 24–30.25 years). Most of the patients were being treated with benzodiazepines at inclusion (86%).
Eleven patients were lost to follow-up. However, we found no statistically significant differences between patients who completed the study and those who did not (Appendix 1, Table S2). At 1-year follow-up, mean (± SD) PANSS scores in patients with first-episode psychosis were as follows: positive subscale 12.69 ± 7.03; negative subscale 17.18 ± 5.89; general pathology 31.67 ± 9.26; and total score 62.51 ± 19.39. Of the patient group, 41% had persistent negative symptoms, and the mean (± SD) GAF score was 61.18 ± 17.68.
A total of 50 healthy controls were included in this study. More than half (56%) were male, and the median age was 26 years (interquartile range 23–30 years). Other clinical characteristics of the sample are shown in Table 1.
Sociodemographic, volumetric and clinical characteristics at baseline
Hippocampal and amygdalar absolute volumes (group means ± SD; patients: left hippocampus 3.88 ± 0.38 mL, right hippocampus 4.09 ± 0.45 mL, left amygdala 1.48 ± 0.24 mL, right amygdala 1.68 ± 0.22 mL; healthy controls: left hippocampus 4.17 ± 0.42 mL, right hippocampus 4.28 ± 0.45 mL, left amygdala 1.67 ± 0.20 mL, right amygdala 1.89 ± 0.25 mL) were comparable to those previously reported in similar clinical populations using the same FreeSurfer method.41–44,46,47
Univariate analysis (Table 1) showed that patients had significantly smaller relative volumes in the left and right amygdala (t = −4.26, p = 0.001; t = −4.32, p = 0.001) compared to healthy controls. Initially, left and right hippocampal relative volumes were significantly lower in patients compared to healthy controls (t = −3.63, p = 0.047; t = −2.13, p = 0.048; Appendix 1, Figures S2 and S3), but after Bonferroni correction these findings were no longer significant (p = 0.047 and 0.048 > 0.05/4 = 0.0125).
Predictors of negative symptoms measured with PANSS at 1-year follow-up
In the univariate analysis (Table 2), PANSS negative subscale scores at 1 year were negatively correlated with baseline serum BDNF levels (r = −0.4; p = 0.012) and with left amygdala relative volume (r = −0.51; p = 0.001) in patients with first-episode psychosis. Conversely, PANSS negative subscale scores at 1 year were positively correlated with PANSS negative subscale scores at baseline (r = 0.41; p = 0.009).
Association of sociodemographic, volumetric and clinical characterics with PANSS negative score and persistent negative symptoms at 1-year follow-up: univariate analysis
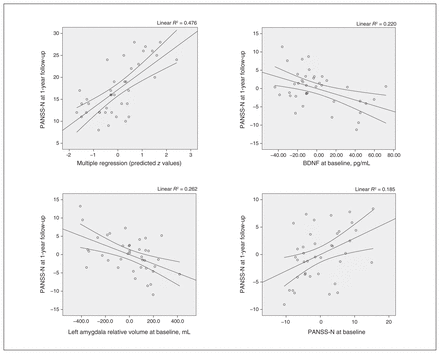
In the multivariate analysis, the model that best predicted PANSS negative subscale scores at 1-year follow-up included the following baseline variables: serum BDNF, PANSS negative subscale scores and left amygdala relative volume, with an R2 of 0.478. This finding confirmed that lower baseline serum BDNF level (95% confidence interval [CI] −0.12 to −0.02; p = 0.011), smaller left amygdala relative volume (95% CI −33.14 to −9.03; p = 0.001), and higher baseline PANSS negative subscale score (95% CI 0.04 to 0.48; p = 0.021) were associated with higher PANSS negative subscale scores at 1-year follow-up in patients with first-episode psychosis (Table 3 and Figure 2). When we conducted the different models adding 1 variable at a time, BDNF explained 15.8% of the variance, baseline PANSS negative subscale score explained 13.2% and left amygdala relative volume explained 18.8%. We also performed a robust regression to confirm that these associations held (with PANSS negative subscale score at 1-year follow-up as a dependent variable and BDNF level, left amygdala relative volume and baseline PANSS negative subscale score as independent variables; Appendix 1, Table S4).
Correlations between PANSS-N scores at 1-year follow-up and BDNF levels, left amygdala relative volume and PANSS-N scores at baseline. BDNF = brain-derived neurotrophic factor; PANSS-N = Positive and Negative Syndrome Scale, negative subscale.
PANSS negative score at 1-year follow-up: linear regression model
We performed an exploratory univariate analysis to assess predictors of PANSS negative subscale subitems at 1-year follow-up (Appendix 1, Table S3). In a multivariate analysis, baseline PANSS negative subscale score was a significant predictor of those subitems. Baseline CDSS score was a significant predictor of N2 (emotional withdrawal; 95% CI = 0.02 to 0.26, p = 0.025) and sex was a significant predictor of N7 (stereotyped thinking; 95% CI = −1.87 to −0.56, p = 0.001).
Predictors of persistent negative symptoms at 1-year follow-up
In the univariate analysis (Table 2), persistent negative symptoms at 1 year were significantly associated with baseline serum BDNF levels (t = 2.93; p = 0.006) and left amygdala relative volume (t = 2.47; p = 0.018) in patients with first-episode psychosis (Appendix 1, Figures S4 and S5).
Based on the findings of the multivariate analysis, the final model included 2 baseline variables: serum BDNF levels and left amygdala relative volume (R2 = 0.533). These results further supported the association of lower baseline serum BDNF levels (OR 0.93, 95% CI 0.88 to 0.98; p = 0.012) and smaller left amygdala relative volume (OR 0.99, 95% CI 0.98 to 0.99; p = 0.010) with the presence of persistent negative symptoms at 1 year in patients with first-episode psychosis (Table 4). We repeated this analysis including chlorpromazine equivalents to control for antipsychotic adverse effects and found no significant differences (Appendix 1, Table S4). When we conducted the different models adding 2 variables each time, BDNF explained 27% of the variance and left amygdala relative volume explained 22.7% of the variance.
Persistent negative symptoms at 1-year follow-up: logistic regression model
Discussion
In this study, we found that lower serum BDNF levels, smaller left amygdala relative volume and more negative symptomatology at baseline predicted more negative symptoms (PANSS negative subscale) at 1-year follow-up. In addition, lower baseline serum BDNF levels and a smaller left amygdala relative volume were predictors of persistent negative symptoms at 1 year. Finally, compared with healthy controls, patients with first-episode psychosis had smaller relative amygdalar volumes and a trend toward smaller hippocampal volumes at baseline.
In our cohort, lower baseline serum BDNF levels predicted higher negative symptoms at 1 year, measured using the PANSS negative symptom subscale or persistent negative symptoms. These findings were consistent with previous reports, which have also found that lower serum BDNF levels are associated with negative symptoms in patients with first-episode psychosis48 and schizophrenia.49,50 Our results were also in line with those of a study that found more severe negative symptoms in patients with first-episode psychosis who were carriers of the Met allele of the BDNF Val66Met polymorphism, a variant that has been associated with decreased BDNF activity.51 Nonetheless, a meta-analysis concluded that decreased peripheral BDNF levels did not appear to be associated with the severity of positive or negative symptoms.52 However, it is important to note that the association between BDNF levels and negative symptoms in that meta-analysis was assessed cross-sectionally and did not take into account possible confounders, such as secondary sources of negative symptoms.
The findings of this study were in agreement with animal studies showing an influence of BDNF on dopamine release in the mesolimbic dopamine pathway,53 which in turn is associated with improved motivational behaviours.54 However, the design of the present study did not allow us to test the mechanisms underlying the association between BDNF and negative symptoms.
Left amygdalar relative volume at baseline predicted both negative symptoms (PANSS negative subscale) and persistent negative symptoms at 1-year follow-up. Previous studies have shown that structural abnormalities in the amygdalar–hippocampal complex are associated with negative symptoms in patients with schizophrenia and first-episode psychosis.10 One study found that patients with first-episode psychosis who presented with persistent negative symptoms had smaller left amygdalar and right hippocampal volumes than patients without persistent negative symptoms.26 Furthermore, several studies have found an association between functional activation patterns in the amygdala and the severity of affective flattening in patients with schizophrenia.55,56
The amygdala plays a key role in emotion and motivational behaviours.57 Alterations in its structure might contribute to the differential expression of negative symptoms. The replicated findings regarding the association between negative symptoms and left amygdala volume in first-episode psychosis26–28 suggest that the lateralization of amygdala volume differences in patients with first-episode psychosis may be an early predictor of negative symptomatology. This finding would be in line with those of some meta-analyses, which reported that amygdala activations are lateralized to the left, particularly for negative symptoms.58,59
Nevertheless, we found no association or mediation effect between negative symptoms and amygdala volume or BDNF levels, as seen in the mediation analysis (Appendix 1, Figure S6). This would suggest that the mechanism by which negative symptoms develop in the case of the amygdala are independent of BDNF levels. As we commented above, BDNF may be associated with negative symptoms through a functional process by increasing dopamine activity in the mesolimbic pathway.53
This mechanism could also explain the lack of a significant association between hippocampal volumes and negative symptoms that one would expect, given the known association between hippocampus and BDNF, and given previous findings. Furthermore, it could also be that in previous studies hippocampal volume was not directly associated with negative symptoms, but rather with other symptoms commonly associated with them, such as cognitive symptoms.60
In our exploratory analyses of the subitems of the PANSS negative subscale, only the total baseline PANSS negative subscale score predicted PANSS negative subscale subitems at follow-up (except for emotional withdrawal and stereotyped thinking, which were predicted by baseline CDSS scores and sex, respectively). Patients with more symptoms of depression at baseline had higher emotional withdrawal scores at 1-year follow-up. Although it would be reasonable to assume that higher emotional withdrawal scores were attributable to an affective disorder, it is important to note that no patients scored 6 or higher on the CDSS at baseline or follow-up. Consequently, this finding for emotional withdrawal could have been because of difficulties in differentiating between depressive and negative symptoms in patients with psychosis, and particularly in those with first-episode psychosis. On the other hand, the fact that none of the other variables were significant predictors of the PANSS negative subscale subitems could have been secondary to the sample size. However, another explanation could be that the total PANSS negative subscale score measures something different than the sum of the subitems.
The variables that predicted negative symptoms on the PANSS negative subscale and persistent negative symptoms at 1 year were highly consistent. This finding was not surprising, given that baseline PANSS negative subscale scores were among the main predictors of PANSS negative subscale scores at 1 year. These findings were consistent with previous reports, which have found that negative symptoms tend to be persistent if they are already present at illness onset, even though their severity may fluctuate over time.10
With this multidimensional approach, we predicted 47.8% of the variance of negative symptomatology measured with the PANSS negative subscale at 1-year follow-up and 53.3% of the presence of persistent negative symptoms. These findings may help guide clinicians from illness onset (e.g., applying more intensive treatments for negative symptoms in patients who are more vulnerable to severe negative symptoms or persistent negative symptoms in the long term). Among such treatments,61 physical activity and add-on antidepressants should be considered, because they have been shown to have beneficial effects on negative symptoms62 and to increase BDNF levels.63 However, future studies should be carried out to test whether an increase in BDNF levels mediates the improvement of negative symptoms with these treatments. Furthermore, the inclusion of other variables should be considered, such has whole-brain gyrification,64 to predict negative symptoms at follow-up even more accurately.
Finally, this study provides additional evidence to the replicated finding that patients with first-episode psychosis have smaller amygdala volumes than healthy controls.65 However, a high proportion of our patients were cannabis users, and that could have affected our results.66
Limitations
This study had several limitations. The first is inherent to all longitudinal studies: not all of the participants completed the entire follow-up. Nonetheless, we found no differences in baseline characteristics between completers and noncompleters, indicating that bias was unlikely. In addition, we did not use a scale to assess extrapyramidal symptoms, although we did take into account antipsychotic adverse effects by including chlorpromazine equivalents in the analyses. Finally, we were unable to assess negative symptoms and their dimensions with next-generation scales, which were not available when the study was initiated.
Despite these limitations, this study had several important strengths, most notably the large sample of antipsychotic-naive patients with first-episode psychosis, a patient profile that is particularly difficult to recruit. Another strength was the long follow-up period (1 year). Finally, the innovative, multidimensional baseline assessment used in this study further strengthened our findings. We included numerous parameters (clinical, neuroimaging and laboratory assessment) to build a robust model for predicting negative symptoms.
Conclusion
Our findings show that several baseline parameters — low serum BDNF levels, decreased left amygdalar relative volume and more negative symptomatology — were predictive of negative symptoms at 1-year follow-up on the PANSS negative subscale (after taking into account secondary sources). Two of those baseline variables — low serum BDNF and a smaller left amygdalar relative volume — were predictive of persistent negative symptoms at 1 year. These findings are highly relevant, because they provide key data to help guide clinicians in developing individualized treatment plans for patients with early-stage disease. However, future studies are needed to replicate these findings and include the different dimensions of negative symptoms, as well as motivation and pleasure and emotional expressivity factors with new generation-scales.67
Acknowledgements
The authors thank the patients and volunteers who made this study possible. They also thank Bradley Londres for professional English-language editing.
Footnotes
Competing interests: None declared.
Contributors: A. Toll and A. Mané designed the study. I. Canosa acquired the data, which L. Blanco-Hinojo, D. Bergé, X. Duran, T. Legido, F. Marmol, V. Pérez-Solà and E. Fernández-Egea analyzed. A. Toll wrote the article, which all other authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
- Received August 7, 2021.
- Revision received October 15, 2021.
- Accepted November 1, 2021.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/