Abstract
Background: Anhedonia is a key symptom of major depressive disorder (MDD). Anhedonia is associated with aberrant reward processing, but whether it might interfere similarly with the neural processing of aversive stimuli, such as monetary loss, remains unknown. We aimed to investigate potential associations between anhedonia and neural response during reward and loss processing in patients with MDD.
Methods: We investigated blood-oxygen-level-dependent response in the orbitofrontal cortex, cingulate cortex, insula and basal ganglia during monetary reward and loss processing in 182 patients with MDD, using a card-guessing paradigm. We measured anhedonia with the Social and Physical Anhedonia Scale (SASPAS), and we tested for the main and interaction effects of SASPAS scores and the experimental condition (reward or loss) in a full factorial model.
Results: We detected a negative main effect of anhedonia, as well as a significant interaction effect of anhedonia and the experimental condition, on orbitofrontal and insular neural response. Post hoc analyses revealed that the interaction was driven by a significant association between higher anhedonia scores and hypoactivation during loss processing. We observed no significant association between anhedonia and neural response during reward processing.
Limitations: This study had a cross-sectional design.
Conclusion: Our findings confirmed that altered neural processing in the orbitofrontal cortex and insula is a neurobiological feature of anhedonic symptomatology in people with MDD. The pronounced association between anhedonia and blunted neural response during loss processing supports a broader concept for the neurobiological basis of anhedonia. Hence, MDD with anhedonic features might be characterized by reduced neural response to external stimuli, potentially because of amotivation.
Introduction
Anhedonia is one of the core symptoms of major depressive disorder (MDD) in addition to low mood, according to the International Statistical Classification of Diseases and Related Health Problems, 10th revision.1 Anhedonic symptoms are highly prevalent in moderate to severe MDD and are associated with treatment-resistant depression.2 Traditional therapies, including antidepressants (e.g., selective serotonin reuptake inhibitors) and psychotherapy, are often unsatisfactory in treating anhedonia,3 leading to poor clinical outcomes in patients with MDD.4–6 Elucidation of the neural basis for anhedonia in MDD is critically important for guiding the development of innovative therapeutic approaches to relieve anhedonic symptoms.
As a common symptom in psychiatric disorders, anhedonia was first defined in the late 19th century as an inability to experience pleasure.7 At a neural level, it has been consistently linked to deficits in reward processing.8 However, in line with more detailed characterization of the reward processing circuit, anhedonia has been reconceptualized in the last decade. Treadway and Zald9 strongly proposed a differentiation between the motivational and consummatory aspects of reward behaviour, but more recent findings have indicated 3 aspects of reward processing that can be impaired in anhedonia. Following a multistep model, intact reward processing consists primarily of motivation to pursue a reward-promising action, an action plan with subsequent goal-directed behaviour, and then a consummatory phase with a hedonic response.10–12 The impaired motivational aspects of the reward processing model13 overlap with the construct of apathy, which is defined as a quantitative reduction in voluntary, goal-directed behaviours.14 Apathy and anhedonia are both common transdiagnostic symptoms in psychiatric and neurologic disorders.15,16
Neuroimaging findings underpin this psychological construct of different steps in reward processing. The orbitofrontal cortex (OFC) and closely connected prefrontal and limbic areas, such as the anterior cingulate cortex (ACC) and the insula, have been identified as key structures in the top–down regulation of reward processing circuits and intact hedonic functioning.17,18 As an area of sensory integration, emotion processing and hedonic experience,19 the OFC serves as a gateway to consciousness and is involved in reward anticipation, motivation and reward valuation.3 The ACC and insula have been implicated in the valuation of reward outcomes.3,20 Interestingly, the OFC and the insula are activated in the processing not only of reward but also of loss, indicating that shared brain regions are involved in responses to both rewarding and aversive stimuli.21–23 These findings are in line with studies on reward and punishment reversal learning.24
In MDD, anhedonia is strongly associated with aberrant reward processing.3,25 The investigation of reward-related brain circuits in MDD using functional imaging techniques is particularly promising for understanding reduced hedonic capacity in patients with depression. Behavioural deficits resulting from anhedonia are evident in all 3 aspects of reward processing (reward liking, wanting and learning), and the neural underpinnings are partially dissociable.25 In MDD, hypoactivation of the striatum is the most robust finding linked to anhedonic symptoms in patients with depression compared to healthy controls, in all aspects of reward processing.26–28 Hypoactivation of the OFC has been consistently detected during the anticipatory phase of reward, indicating motivational deficits because of anhedonia.25 In a machine-learning attempt to identify the biological subtypes of MDD through patterns of brain function in resting-state functional MRI, alterations in frontostriatal and orbitofrontal connectivity were also correlated with anhedonia.29
Unlike the well-established relevance of altered reward processing in MDD, up to now only a few studies have focused on loss processing and its role in depressive symptomatology — particularly anhedonia. Of the few existing studies on loss processing in MDD, most have pointed to decreased activation in structures of the prefrontal cortex.30–32 However, these studies focused on depression risk or symptomatology in adolescents rather than adults, and were further limited by small sample sizes (33 participants or fewer).
To address this gap in the literature, we aimed to investigate the potential associations between anhedonia and neural response during reward and loss processing in a well-powered sample of patients with moderate to severe depression and graduated levels of anhedonia. We defined the OFC, cingulate cortex, insula and basal ganglia as the region of interest. In line with a vast literature on reward processing in MDD, we expected to replicate functional MRI findings showing hypoactivation of the reward-related areas associated with anhedonia, but we analyzed alterations during loss processing using an exploratory approach because pre-existing findings are scarce.
Methods
Participants
Our study sample comprised 182 patients with MDD. At the time of recruitment, all patients were receiving inpatient treatment at the University Hospital of Münster because of a depressive episode.
Common exclusion criteria were a history of neurologic illness, a medical condition (e.g., cancer, chronic inflammatory or autoimmune disease, infection), head trauma or unconsciousness, alcohol or substance dependence, a psychotic disorder, previous electroconvulsive therapy, and contraindications for MRI.
All participants had normal or corrected-to-normal vision, as well as adequate knowledge of German and cognitive abilities (verbal IQ > 80 on a multiple-choice vocabulary intelligence test33). All participants received financial compensation. The study was approved by the institutional review board of the medical faculty of the University of Münster. All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. We obtained written informed consent from all participants.
All participants underwent a structured clinical interview based on the Diagnostic and Statistical Manual of Mental Disorders, 4th edition,34 administered by trained raters. We verified all clinical diagnoses of MDD and confirmed that all participants had current moderate to severe major depression with no history of (hypo)manic episodes. Psychiatric comorbidities such as anxiety, eating and somatoform disorders (except for bipolar disorder, alcohol and substance dependence and psychotic disorders) were not used as exclusion criteria.
Clinical and medical assessment
We administered the Beck Depression Inventory35 to assess participants’ current depressive symptoms; all participants completed a German version of the 21-item self-report inventory.36 Trained raters also assessed depressive symptoms using the Hamilton Rating Scale for Depression.37 We measured social and physical anhedonia using a validated German version of the Chapman Scales for Physical and Social Anhedonia (SASPAS).38,39
Most patients were taking medication at the time of scanning. To evaluate the potential effects of psychotropic medication, we recorded the type of medication and dose to compute a medication index by applying a strategy described in our previous work.40 Each psychotropic medication was coded as absent (0), low (1; equal to or lower than an average dose) or high (2; greater than an average dose), relative to the midpoint of the daily dose range recommended by the Physician’s Desk Reference.41 We calculated a composite measure of total medication load for each participant, reflecting the dose(s) and variety of medications taken, by summing the medication codes.
We preprocessed and further analyzed the data using statistical parametric mapping software (SPM12, Welcome Department of Cognitive Neurology; www.fil.ion.ucl.ac.uk/spm;RRID:SCR_007037). To identify univariate outliers, we calculated Z scores for SASPAS and medication load index, with a threshold of |Z| ≥ 3. We performed bivariate outlier detection by calculating Mahalanobis distance with a threshold of p < 0.001.
Stimulus materials and procedure
To detect brain activity associated with monetary reward and loss processing, we employed a modified common card-guessing paradigm42,43 as used recently by our group.44
The pseudorandom block-design paradigm consisted of 9 blocks: 3 “win” blocks (1, 4 and 7), 3 “lose” blocks (2, 5 and 8) and 3 control blocks (3, 6 and 9). Each block consisted of 5 trials. During each trial, participants were presented with a card and had 3 s to guess whether its value was higher or lower than 5. After the participant had made their choice, the numerical value of the card was shown for 0.5 s, followed by appropriate feedback for an additional 0.5 s (red down arrow for negative feedback, green up arrow for positive feedback). Whenever they received positive feedback, participants were asked to confirm the gain via a button press. Between trials, a crosshair was presented for alternating durations — 1.5 s for consecutive odd-numbered trials across the entire paradigm (i.e. first, third, fifth, etc.) or 2.5 s for consecutive even-numbered trials (i.e. second, fourth, sixth, etc.) — resulting in total trial lengths of 5.5 and 6.5 s, respectively.
During the 3 “win” blocks, participants received predominantly positive feedback (4 trials, 80% correct); during the 3 “lose” blocks, they received predominantly negative feedback (4 trials, 80% false). Participants were told that each time they received positive feedback, they would be given 1 euro; each they received negative feedback, 50 cents would be deducted. The “win” and “lose” blocks were interleaved with 3 control blocks, during which participants were asked to press a button at random when an x was presented (3 s), followed by an asterisk (0.5 s), a yellow circle (0.5 s) and a crosshair (1.5 s for odd-numbered trials and 2.5 s for even-numbered trials). All blocks were preceded by an instruction (3 s), yielding a total length of 32.5 s for odd-numbered blocks, 33.5 s for even-numbered blocks and 296.5 s for the entire task.
Participants were told that their financial compensation would be based on their performance on the card game; however, they were unaware that outcomes were standardized (3 each of “win,” “lose” and “control” blocks). In fact, compensation was fixed, and after the assessment, all participants were informed that they would receive 10 euros.
Functional MRIs
Data acquisition and preprocessing followed standardized protocols as described in our previous work.40,44
We acquired T2* functional data using a 3 T scanner ( Gyroscan Intera 3 T, Philips Medical Systems) and a single-shot echo planar sequence. Parameters were selected to minimize distortion in the region of interest while retaining adequate signal-to-noise ratio and T2* sensitivity. We acquired volumes consisting of 34 slices (matrix 64 × 64; resolution 3.6 mm × 3.6 mm × 3.6 mm; repetition time 2.1 s; echo time 30 ms; flip angle 90°). Slices were tilted 25° from the anterior–posterior commissure line to minimize dropout artifacts in the mediotemporal and orbitofrontal regions.
The paradigm presentation was projected to the rear end of the scanner (Sharp XG-PC10XE with additional high-frequency shielding). During the experiment, participants lay supine in the MRI scanner with the response box in their right hand.
Preprocessing of our functional data included realignment, unwarping and spatial normalization to Montreal Neurological Institute space, as well as smoothing with a Gaussian kernel of 6 mm full width at half-maximum.
To isolate neural response during the different blocks (win, lose or control), we modelled the onsets and durations of the experimental conditions using a canonical hemodynamic response function. We did this in the context of the general linear model, including corrections for serial correlations and applying a high-pass filter of 128 s to remove low-frequency noise.
For each participant, we conducted first-level analyses yielding the contrast-images “reward > control” and “loss > control.” We checked the study sample for excessive head movement, and all participants fell below the threshold values of 3 mm, 3° or both.
Statistical analysis
We carried out the following steps for second-level analyses of the contrast images “reward > control” and “loss > control.” Before testing the association between anhedonia and reward or loss processing, we explored the main effect of the condition (reward or loss) at a whole-brain level using an uncorrected threshold (p < 0.001) to verify neural activation during the paradigm, especially in our region of interest.
To address our hypothesis that anhedonia would be associated with altered reward and loss processing, we used a full factorial model, with 1 factor comprising 2 levels (“reward > control” and “loss > control”) and SASPAS scores as covariates. We identified age, sex and medication load index as probable confounding variables and included them as additional covariates. We tested for a main effect of medication load index as a potential major confounder, with age and sex as covariates. We also performed an unpaired t test comparing patients who were currently taking antipsychotic medication (n = 68) and patients who were antipsychotic-naive (n = 115) to examine potential group differences as a result of medication status.
We performed anatomic labelling using the AAL-Toolbox.45 Using a region-of-interest approach, we restricted analyses to 1 mask comprising the OFC, insula, cingulate cortex, caudate nucleus, putamen and nucleus accumbens using the IBA SPM 71 atlas46 (implemented in the WFU pickatlas; http://fmri.wfubmc.edu/software/PickAtlas,RRID:SCR_007378).
We obtained significance thresholds for multiple testing at the cluster level by using threshold-free cluster enhancement (TFCE) as a nonparametric approach,47 implemented in the TFCE toolbox (http://dbm.neuro.uni-jena.de/tfce, version 167) with a minimum cluster volume threshold of k ≥ 30. We established a conservative family-wise error (FWE)–corrected threshold of p < 0.05 obtained by 5000 permutations per test.
Results
Descriptive statistics
For detailed sociodemographic and clinical characteristics of our study sample, see Table 1. We detected 1 univariate outlier for medication load index (Z = 3.57). However, after carefully reviewing the plausibility of the medication index, we did not exclude the patient. Following calculation of Mahalanobis distance, we detected no significant bivariate outliers below the threshold of p < 0.001.
Participant sociodemographic and clinical characteristics (n = 182)
Main effect of condition
Whole-brain analysis of the main effects of the reward and loss conditions compared to the control condition revealed robust activation of blood-oxygen-level-dependent (BOLD) response in widespread regions, including the OFC, cingulate cortex, insula and basal ganglia (for further details, see Appendix 1, Figure S1, available at www.jpn.ca/lookup/doi/10.1503/jpn.210180/tab-related-content).
Main effect of anhedonia
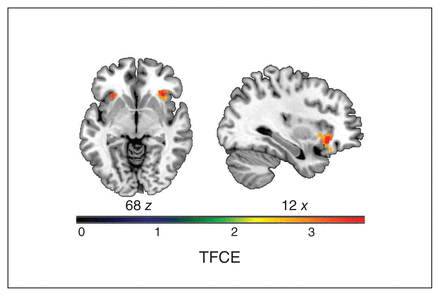
We observed a significant main effect of anhedonic symptoms on BOLD response in the insula, OFC, ACC and basal ganglia (Table 2). Post hoc analysis employing multiple regression models revealed significantly attenuated BOLD response in the right insula and OFC (x, y, z = 32, 26, −6; t357 = 3.79; TFCE = 283.99; pFWE = 0.027; k = 121 voxels) and in the left insula (x, y, z = −30, 22, −4; t357 = 3.94; TFCE = 248.74; pFWE = 0.037; k = 34 voxels) with increasing SASPAS scores as covariates (Figure 1). We detected no significant increase in BOLD contrast at the applied threshold.
Results from multiple regression analysis of the effect of anhedonia on BOLD response during a loss condition, displaying clusters in the bilateral insula and orbitofrontal cortex. Axial (z = 68) and sagittal (x = 12) views of the region of interest. Colour bar depicts TFCE scores (pFWE = 0.05 after TFCE). BOLD = blood-oxygen-level-dependent; FWE = family-wise error; TFCE = threshold-free cluster enhancement.
Main effect of anhedonia (Social and Physical Anhedonia Scale)*
Interaction effect of anhedonia and condition
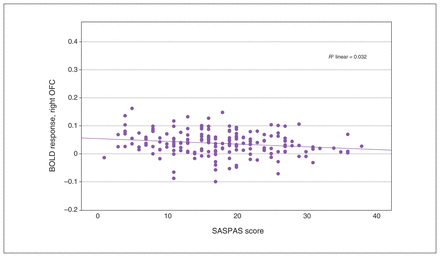
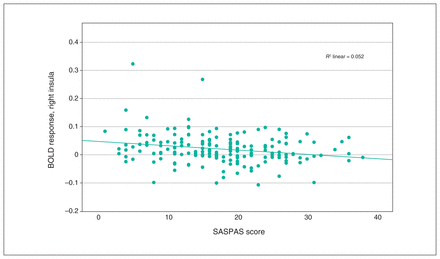
We observed a significant interaction effect of anhedonia and condition in the OFC, insula, ACC and basal ganglia (Table 3). Post hoc analyses employing multiple regression models revealed a significant decrease in BOLD response associated with anhedonia in the loss condition, in the right insula and the right superior and inferior OFC (x, y, z = 34, 24, −8; t357 = 3.80; TFCE = 310.41; pFWE = 0.024; k = 227 voxels), as well as in the left insula and left inferior OFC (x, y, z = −30, 22, −4, t357 = 3.85, TFCE = 260.20, pFWE = 0.038, k = 43 voxels; x, y, z = −36, 24, −14, t357 = 3.56, TFCE = 241.90, pFWE = 0.045, k = 15 voxels; Figure 2 and Figure 3). We detected no association between anhedonia and BOLD response at the applied threshold in the reward condition.
Scatterplot depicting peak BOLD response in the right OFC, from multiple regression analysis with anhedonia scores as covariates. Pearson correlation: r = −0.176, p = 0.016. BOLD = blood-oxygen-level-dependent; OFC = orbitofrontal cortex; SASPAS = Social and Physical Anhedonia Scale.
Scatterplot depicting peak BOLD response in the right insula, from multiple regression analysis with anhedonia scores as covariates. Pearson correlation: r = −0.227, p = 0.002. BOLD = blood-oxygen-level-dependent; SASPAS = Social and Physical Anhedonia Scale.
Interaction analysis: condition (“reward” or “loss”) × anhedonia (Social and Physical Anhedonia Scale)*
Main effect of medication load index
In our region of interest and at the applied threshold, we detected no significant main effect of medication load index on BOLD response during the reward or loss conditions.
Group comparison
In our region of interest and at the applied threshold, we observed no significant difference between patients currently taking antipsychotic medication and patients who were antipsychotic-naive in terms of BOLD response during the reward and loss conditions.
Discussion
With the present study, we provide evidence for blunted insular and prefrontal neural response during reward and loss processing as a general function of anhedonia in patients with MDD. Interestingly, we detected an interaction effect between condition (reward or loss) and anhedonic symptoms that was driven by a pronounced association between anhedonia and reduced neural response during loss processing.
We provide novel evidence that loss perception seems to be processed by the insula and the OFC, overlapping with reward processing regions. However, our findings demonstrate an association between decreased neural response and anhedonia during loss processing that we did not detect for reward stimuli. These differential findings are notable, because hypoactivation of reward-related brain regions such as the OFC and ventral striatum is a well-replicated finding in patients with MDD compared to healthy controls.27
Our results highlight the need to reconsider the concept of anhedonia in MDD, not only by decoding different steps in reward processing, but by including loss perception. Patients with depression and pronounced anhedonia seem to be characterized by a general decrease in sensitivity to environmental stimuli, possibly because of motivational deficits.
Amotivational behaviour is associated with anhedonic features — as is apathy. Both symptoms seem to share neurobiological mechanisms, as proposed by Husain and Roiser.48 Key brain areas of motivation are the medial OFC, ACC and basal ganglia as part of a frontostriatal circuit.48,49 The OFC has been associated with processing the subjective incentive value of stimuli.50,51 The medial prefrontal cortex and insular cortex are part of a stimulus evaluation or salience network that is coactive upon rewarding or punishing stimuli.52–54 Reduced BOLD response in these regions might lead to less motivated behaviour as both information and incentive value processing is impaired, potentially leading to clinical symptoms such as anhedonia.53
The results of our study highlight aberrant functioning of the OFC and insular cortex in patients with MDD and hint at an overlap of anhedonia and apathy in MDD. In addition to measuring consummatory anhedonia, the SASPAS questionnaire we used focuses on anticipatory anhedonia, implicating a reduced motivation to engage in social or physical interaction.55 Still, to further characterize not only the associations between anhedonia and apathy but also their differences, replication of our findings using other anhedonia measurement tools and (more importantly) apathy-specific questionnaires is needed.
Clinical anhedonia has long been associated with poor outcomes and a chronic course of disease.56 Compared to other symptoms such as low mood, sleep disturbances or changes in appetite, anhedonia is a depression symptom that is difficult to treat, especially because it is not sufficiently targeted by conventional antidepressants or psychotherapy.3,57,58 Treatment response is often measured by improvement in emotional deficits, disregarding persistent anhedonic features.59 To better characterize subgroups of depression, it has been proposed that anhedonia be a psychopathological endophenotype of depression in need of novel therapeutic approaches.48,60,61 Our imaging results could support this notion of a specific subgroup of acutely depressed patients with severe anhedonic symptoms, who show the most pronounced blunting of BOLD response, particularly upon aversive stimuli. In terms of diagnostics, decreased insular and orbitofrontal neural activation, in line with findings on orbitofrontal volume reduction in people with depression, could be an imaging state marker for depression with severe anhedonic symptoms as a predictor of treatment resistance.62,63 Future studies might investigate the potential utility of this imaging marker for treatment stratification. Clinical research might also benefit from the inclusion of such imaging results in the evaluation of anhedonia-specific interventions, which should not only include improvement of the hedonic experience but also tackle motivational deficits. To strengthen these hypotheses, a longitudinal study design is needed to clarify whether symptom decline or complete remission leads to normalization of activation patterns. Otherwise, persisting neural abnormalities of reward and loss processing might be of predictive value, especially if they are acquired early in adolescence.
Scrutinizing anhedonia sheds light on the transdiagnostic challenges we face in psychiatric research. Anhedonia is clearly a core symptom of MDD, but it also a common negative symptom in schizophrenia. In addition, it is often apparent in patients with long-term substance abuse or neuropsychiatric disorders such as Parkinson disease and chronic pain.15,64 We should bear this in mind when we identify new endophenotypes of depression, especially when designing novel therapeutic approaches and treating patients with (neuro)psychiatric comorbidities.
Limitations
The results of our study provide novel insights into the associations between reward and loss processing in MDD and anhedonic symptoms, but several limitations should be acknowledged. First, the cross-sectional design of our study did not allow us to conclude whether the neural alterations associated with anhedonia that we observed were state- or trait-dependent. Future longitudinal studies are needed to answer these questions. Second, the lack of a healthy control group left the question open as to whether our results were specific to a sample of patients with depression or correlational with anhedonia in general. Furthermore, the applied reward and loss processing paradigm was based on monetary compensation as a secondary reward. Perception of rewarding and aversive stimuli is much more complex in the everyday experiences of patients with MDD; future studies based on ecologically valid paradigms are warranted to examine the generalizability of our findings. Finally, the SASPAS questionnaire we used does not provide subscales to differentiate between the motivational and consummatory aspects of anhedonia.
Conclusion
Our well-powered study supports the hypothesis of altered neural processing as a neurobiological feature of anhedonic symptomatology in patients with MDD, possibly defining a subphenotype. The pronounced neural blunting we observed during loss processing in patients with anhedonia might be a promising neural target for future studies investigating innovative psychopharmacological or psychotherapeutic interventions. In a broader perspective, our results add reduced neural activity with aversive stimuli to the detailed concept of anhedonia in patients with MDD. Future research should focus on motivational deficits in patients with severe anhedonia and investigate an overlap with apathy using a variety of aversive stimuli.
Footnotes
↵* These authors contributed equally to the present work and should both be regarded as senior authors.
Competing interests: J. Goltermann received a Society of Biological Psychiatry 2022 travel Award. No other competing interests declared.
Contributors: U. Dannlowski designed the study. K. Dohm, J. Goltermann, M. Richter. V. Enneking and M. Lippitz acquired the data, which L. Steinmann, J. Repple, M. Mauritz and N. Opel analyzed. L. Steinmann wrote the article, which K. Dohm, J. Goltermann, M. Richter, V. Enneking, M. Lippitz, J. Repple, M. Mauritz, U. Dannlowski and N. Opel reviewed. All authors approved the final version to be published, agree to be accountable for all aspects of the work and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
Funding: This work was supported by the German Research Foundation (U. Dannlowski, grant nos. DA1151/5-1, DA1151/5-2 and SFB-TRR58, projects C09 and Z02); the Interdisciplinary Centre for Clinical Research (IZKF) of the Medical Faculty the University of Münster (U. Dannlowski, grant no. Dan3/012/17; N. Opel, grant no. SEED 11/18); and the Deanery of the Medical Faculty of the University of Münster (K. Dohm, grant no. KO121806). The authors also acknowledge support from the Open Access Publication Fund of the University of Münster.
- Received October 19, 2021.
- Revision received April 16, 2021.
- Accepted May 8, 2022.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/