Lower costs of both genotyping and magnetic resonance imaging (MRI) acquisition have provided an unprecedented opportunity to understand how genetic factors shape brain morphology. These findings, in turn, may help us better understand the pathophysiology of various neurologic and mental health disorders that pose a significant global disease burden.1
Genome-wide association studies (GWASs) scan through millions of common genetic variants to find loci that are significantly linked to phenotypes of interest. These common variants, also known as single nucleotide polymorphisms (SNPs), are found in a sizable fraction (i.e., 1 % or more) of the population. The GWAS approach has helped the field move beyond classic candidate gene studies (e.g., COMT) and associations with brain morphology and connectivity that permeated the field a decade ago but have since been shown to have poor reproducibility.2 Multiple GWASs have recently been published to better understand the genetic architecture of various brain features,3,4 such as MRI-derived cortical features,5–8 subcortical structures9 and white matter features.10,11 The cortex in particular has been of utmost interest to neuroscientists given that its unique expansion in humans has coincided with the emergence of complex behaviours.12
It is well known in neuroimaging studies that different brain atlases, or the way different regions of interest are defined, can have a significant impact on findings. This methodological choice is equally important for genomic studies integrating brain imaging data. Many previous cortical GWASs have been successful in identifying novel genetic variants underlying morphological features of the brain, but typically define brain regions based on anatomical (e.g., sulcal/gyral) patterns. A recent publication from our group harnessed genetic information to draw regional brain boundaries, potentially improving discovery of genetic loci linked to the cortex compared with previous studies.5
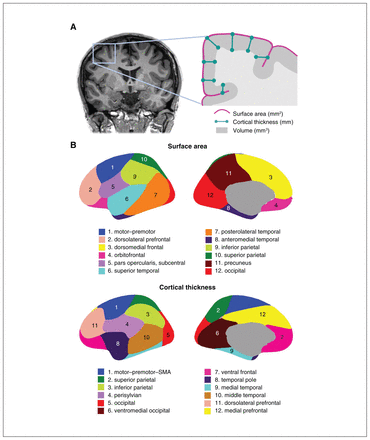
These genetically informed brain atlases,8,13,14 comprising 12 surface area and 12 thickness regions (Figure 1), conform to known patterns of genetically mediated cortical patterning during neurodevelopment and have been independently replicated.15,16 Genetic cortical patterning includes features such as the anterior–posterior axis, describing developmental effects of brain expansion or surface area, as well as identification of functional brain regions underlying complex traits in humans, such as the dorsolateral prefrontal cortex and its role in working memory. These atlases also show striking concordance between the genetic divisions of the brain and the boundaries of various functional regions that clinicians are already familiar with in their neuroanatomical assessment of patients (e.g., orbitofrontal cortex and its links to depression, anteromedial temporal cortex and epilepsy). Our aim in this editorial is to help clinicians sift through the wealth of data that GWAS provides, using the clinically meaningful anatomical patterns and genes described in our latest work as an example.
(A) Coronal MRI slice and inset schematic outlining the relationship between surface area, cortical thickness, and volume. (B) Genetically informed brain atlases of surface area and cortical thickness. Note: these brain regions are displayed on a smooth version of the brain that does not delineate sulcal/gyral patterns. MRI = magnetic resonance imaging; SMA = supplementary motor area.
Neuroanatomy — genetic variability shapes regions of the brain differently
The genetically based atlas of the human cortex depicted in Figure 1B,8 initially derived from the MRI data of more than 400 twins using a data-driven fuzzy clustering algorithm,13,14 set the foundation for the discovery of 440 genetic loci (and nearly 800 significant loci in a meta-analysis combining total samples) underlying cortical surface area and thickness of the human brain, which we will hereafter refer to as cortical morphology for brevity. As mentioned, some of the boundaries in our genetically based atlas follow traditional gyral-based patterns (e.g., inferior and parietal area), whereas some diverge (e.g, no distinction between dorsomedial frontal and orbitofrontal regions for the area atlas).13 Given the nontrivial nature of mapping loci to genes owing to intergenic loci and the complex correlated structure between SNPs, multiple workflows have been recommended to improve accuracy of identifying plausible genes. One such gene-mapping strategy is based on positional information with the MAGMA tool;17 another method additionally incorporates gene expression and other epigenomic annotations (e.g., FUMA18). In our study, these 2 approaches yielded 575 and 915 genes, respectively, associated with cortical morphology.5 Single-cell data profiling accessible chromatin can also add to gene mapping efforts. These genes are then annotated for significant biological pathways to help with the functional interpretation of significant loci that have identified pathways related to development and morphogenesis in cortical GWASs.5,6,8 It is important to bear in mind that these mapped genes are putative causal genes only, given that GWAS associations do not directly yield a gene target or mechanistic insight.19,20 Mapping strategies to identify causal genes from GWAS has proven to be challenging, but is an active area of research, for instance by leveraging gene expression data to bring us a step closer to causal genetic mechanisms.19,21,22
Collectively, cortical phenotypes have moderate to high heritability (area 0.27; thickness 0.20 on average8), compared with other commonly studied phenotypes in humans (mean heritability of 0.22 across 28 complex phenotypes23). Heritability is an estimate of the amount of variation in a phenotype that can be explained by genetic differences. Specifically, GWAS typically uses measures of SNP-based heritability to quantify the overall contribution of the additive effects of all SNPs (capturing a fraction of twin heritability). Given that subtle alterations in structural brain measures may reflect endophenotypes of neuropsychiatric disorders,24,25 uncovering the genetic underpinnings of brain structure can, in turn, provide important clues into neuropsychiatric conditions.
Pathophysiological implications — genes related to brain structure/development are also related to disease
There is notable overlap between disease-related genes reported previously and genes associated with cortical phenotypes. Our recent work8 nonexhaustively included these links based on their genetic discovery in a supplementary table based on the Genetics Home Reference database. This is a data source similar to Online Mendelian Inheritance in Man (OMIM) in which many gene–phenotype associations are from monogenic disorders or conditions attributable to genes with large effects. Cortical GWAS are often conducted on nonclinical data; thus, discovered loci reflect genetic effects on normative variation of the brain. However, these loci could be pleiotropic between brain structure and diseases or point toward a continuous spectrum of genetic effects from normal neurodevelopment to aberrant phenotypes. We highlight some key findings for disorders commonly encountered by clinicians, and add some examples from recent psychiatric GWASs in Table 1. Although experimental validation of the causal impact of many of these genes is still needed, several have been tied to biological processes involving brain development, for instance through the role of PTCH1 in SHH-mediated signalling and regulation of neurodevelopment.26
Examples of genes mapped from significant loci associated with cortical phenotypes8*
Spontaneous mutations with congenital neuroanatomical anomalies also offer a natural opportunity to glean insights into the genetic control of cortical development. Focal polymicrogyria is an example of a genetically determined cortical malformation that develops only in specific regions of the cortex, strongly supporting the notion of regional genetic influences on cortical area development.27 Among the identified genes in our recent work,8 TUBB was linked to posterolateral temporal surface area. Tubulin gene subclasses are widely expressed in the developing brain,28 and have also been linked to cortical morphology and asymmetry in previous GWASs.6,29 There was also genetic overlap with holoprosencephaly, a common developmental defect where the cerebral hemispheres fail to separate properly.30 Holoprosencephaly has been attributed to a gain-of-function mutation or duplication of PTCH1, a gene found to be associated with occipital, posterolateral temporal, dorsomedial and orbitofrontal cortical morphology5,8,31 as well as microstructure of the uncinate fasciculus.10 Additionally, ZIC1 and ZIC4 have been mapped to the surface area of frontoparietal cortices.5,8 Both of these genes in the zinc finger protein family have been previously associated with holoprosencephaly and forebrain and pontocerebellar anomalies,32 notably in Dandy–Walker malformation.33
Beyond structural malformations, the complex genetic signal uncovered in cortical GWASs vary by brain region and may help bridge the knowledge gap between regional cortical morphology and diseases. This is particularly relevant for neurodevelopmental disorders with subtle neuroimaging features, such as autism spectrum disorder (ASD). We highlight an example in Table 1 linking ASD to 2 genes, ASH1L and SYNGAP1. These genes were mapped to the surface area of 3 different regions, suggesting they may affect some cortical regions more strongly than others. Intriguingly, SYNGAP1 has been causally linked to various neurodevelopmental disorders, including ASD.34 We also found reduced anteromedial temporal surface area to be putatively causally linked to ASD,8 comprising regions that have also been shown to have reduced brain volume in SYNGAP1 heterozygous mutant mouse models.34 It should be acknowledged that the nonsignificant cortical–ASD pairs in our work may not be true negatives given that, with increasing sample sizes, it is possible other regions may become significant. However, the 3 regions we do find associated with ASD likely have quite large effects and warrant further exploration to help inform the structural basis of functional impairments in ASD and related disorders.
We and others5,8 have observed only some significant genetic correlations between cortical morphology and neurologic, as well as psychiatric, disorders. This may be in part owing to low statistical power, the cumulative gene–environment interactions underlying their complex etiology,35 as well as methodological limitations given that the genetic correlation calculation averages over numerous SNPs. Other statistical methods focusing on specific genomic compartments (e.g., regulatory regions) could be beneficial. These genetic correlations also excluded the major histocompatibility complex (MHC), a genomic region contributing to psychiatric disorders and cortical measures. Apart from methodological choices, some psychiatric disorders, such as major depressive disorder, may have subtle neuroanatomical phenotypes or are linked more strongly to functional changes in neural circuitry, which might explain the challenge in observing their genetic overlap with gross neuroanatomical phenotypes.36 More evidence has emerged recently, however, for structural abnormalities in psychiatric disorders.3 Discovering the biological basis of these disorders is a problem psychiatry faces at large, given the blurred boundaries and heterogeneous subgroups within current diagnostic definitions alongside the polygenic nature (i.e., contribution of many genetic variants each with tiny effects) of these disorders.37 GWAS approaches integrating brain imaging data may have important implications for understanding their pathogenesis, as well as moving toward clinical subtyping efforts, particularly for disorders with neurodevelopmental origins. Methodological advances and larger sample sizes could also help further elucidate the genetic overlap between cortical morphology and psychiatric disorders. For instance, in our latest work we apply Mendelian randomization21 and find a putative causal association between anteromedial temporal area and ASD.8 Multivariate techniques or polygenic scoring hold promise in deciphering the shared genetic architecture between neuroimaging and psychiatric phenotypes.38–40
Future directions
Altogether there is a clear connection among genes, brain morphology and brain-related disorders in a biologically meaningful way. Recent brain imaging GWASs have provided new evidence to associate regional brain morphology with neuropsychiatric diseases. Genetic testing has already been successfully integrated into clinical care in several cases for severe neurodevelopmental disorders,42 such as FMR1 for Fragile X.43 However there is a need to merge our understanding of such disorders with the genetic architecture of the brain — a gap that recent work begins to address.5,8,10,31 Similarly, for common polygenic diseases, it may be fruitful to consider polygenic scores, where individual small SNP effects are aggregated into a single score for an individual based on their genotype. Combining polygenic scores derived from GWAS results of brain phenotypes with polygenic risk scores for psychiatric/neurologic disorders may offer more information to predict an individual’s risk for a given clinical variable.
Careful consideration of brain atlases integrated into genomic work is warranted. We have highlighted one set of genetically informed atlases of cortical morphology.13,14 Such genetically informed atlases require further replication and improvement, which would be a worthwhile endeavour for the field of imaging genetics. Following the successful efforts of the Human Connectome Project (HCP)44 to incorporate multimodal imaging into atlas generation, as well as evidence for common genetic variants contributing to white matter and functional imaging phenotypes,45,46 we encourage researchers working with different imaging modalities (e.g., diffusion/functional MRI and positron emission tomography) to explore genetically based parcellations and how they could inform associations with psychiatric disorders.
Beyond the brain imaging measures themselves, it will be important for the field to also replicate atlases in different populations. For instance, the gross cortical patterning captured by the genetic atlases we define largely remain the same with age,47,48 but the extent to which fine-scale regional boundaries differ by factors such as age, sex or ancestry still remain unknown. Further, probabilistic methods (e.g., “fuzzy” atlases) can be employed in capturing inter-subject variations or likelihood of regional boundaries.14,49–51 One of the common denominators of these proposed methods is the availability of large sample sizes.52 Initiatives such as those led by the UK Biobank53 and ENIGMA52,54 are moving in that direction to increase imaging genetic samples, and ongoing harmonization work remains crucial to address the heterogeneity present in multisite studies and meta-analytic approaches.
Recently discovered genomic loci and mapped genes provide a valuable resource for necessary future experiments and clinical studies, with the overarching goal of assisting clinically in evidence-based, personalized anticipatory surveillance and management strategies for patients. It would be beneficial for clinicians to be equipped with adequate resources and educational training to remain up to date with the latest research advances, including results coming from neuroimaging, GWAS and rare variant studies.55 This in turn would set the foundation for continued and necessary discourse and collaboration between clinicians and researchers working in imaging genetics to enhance translational work. The intersection of genes associated with both disorders of interest and their brain endophenotypes, such as the cortical phenotypes we describe, may have important implications for understanding the pathophysiology of these disorders and ultimately, for advancing precision medicine.
Acknowledgments
The work and authors are supported by the National Institutes of Health under R01MH118281, R56AG061163 (PI: C.-H. Chen). C. Makowski is also supported by the Canadian Institutes of Health Research (CIHR) and the Kavli Institute for Brain and Mind (KIBM).
Footnotes
The views expressed in this editorial are those of the author(s) and do not necessarily reflect the position of the Canadian Medical Association or its subsidiaries, the journal’s editorial board or the Canadian College of Neuropsychopharmacology.
Competing interests: None declared.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/