Abstract
Background: The pathophysiology of psychosis is complex, but a better understanding of stimulus binding windows (BWs) could help to improve our knowledge base. Previous studies have shown that dopamine release is associated with psychosis and widened BWs. We can probe BW mechanisms using drugs of specific interest to psychosis. Therefore, we were interested in understanding how manipulation of the dopamine or catecholamine systems affect psychosis and BWs. We aimed to investigate the effect of dexamphetamine, as a dopamine-releasing stimulant, on the BWs in a unimodal illusion: the tactile funneling illusion (TFI).
Methods: We conducted a randomized, double-blind, counterbalanced placebo-controlled crossover study to investigate funnelling and errors of localization. We administered dexamphetamine (0.45 mg/kg) to 46 participants. We manipulated 5 spatial (5–1 cm) and 3 temporal (0, 500 and 750 ms) conditions in the TFI.
Results: We found that dexamphetamine increased funnelling illusion (p = 0.009) and increased the error of localization in a delay-dependent manner (p = 0.03). We also found that dexamphetamine significantly increased the error of localization at 500 ms temporal separation and 4 cm spatial separation (pinteraction = 0.009; p500ms|4cm v. baseline = 0.01).
Limitations: Although amphetamine-induced models of psychosis are a useful approach to understanding the physiology of psychosis related to dopamine hyperactivity, dexamphetamine is equally effective at releasing noradrenaline and dopamine, and, therefore, we were unable to tease apart the effects of the 2 systems on BWs in our study.
Conclusion: We found that dexamphetamine increases illusory perception on the unimodal TFI in healthy participants, which suggests that dopamine or other catecholamines have a role in increasing tactile spatial and temporal BWs.
Introduction
People integrate multiple stimuli over space and time to form unified percepts of objects and events,1 and alterations in integration have been proposed as being important for a range of clinical disorders that experience alterations in perception.2,3 Stimulus binding windows (BWs) reflect the temporal and spatial intervals over which stimuli from multimodal or unimodal sensory systems are to be associated with one another and bound into a single perceptual entity. Values for BWs depend on a variety of parameters: the modalities of the stimuli,4–6 the order of multimodal stimuli,4–6 age,7–10 peripheral sensory loss such as vestibular hypofunction,11 and psychiatric diagnoses such as psychotic illnesses, autism and attention-deficit/hyperactivity disorder.3,12–20
Illusory procedures have been used to investigate body perceptions in people with schizophrenia with passivity symptoms, which are characterized by disturbances in core body representations including body image and body schema.12–14 People with schizophrenia also have wider BWs, and dopamine is strongly implicated in schizophrenia. Like the experience of touch feel illusion or body ownership illusion in rubber hand illusion (RHI) tests in people with schizophrenia and their offspring,17,21 administration of dexamphetamine to healthy participants increases illusion strength in RHI and widens temporal BWs.22 However, the exact role of dopamine in illusory perception and BWs is poorly understood. One way of studying the role of dopamine23,24 and the relation between dopamine and the illusion in healthy people is to use drugs that increase extracellular dopamine levels and dopamine transmission,22 as several drug models of schizophrenia have suggested.25,26 The amphetamine model of schizophrenia is one of the proposed approaches27–30 that is widely used owing to the known pharmacology, low cost, high availability and well-understood safety profile in humans and animals.31,32 Amphetamine increases the synaptic levels of monoamines (dopamine, noradrenaline and serotonin) by increasing their release through a reversal of the monoamine transporters, primarily the catecholamine transporters.33,34 For example, amphetamine increases the synaptic level of dopamine by increasing its release through a reversal of the dopamine transporter.35,36 Dexamphetamine (d-amphetamine) is more potent than l-amphetamine (3- to 10-fold) or a racemic mixture of l- and d-amphetamine.37 It can increase dopamine release, making its dopaminergic effects equipotent with its effects on noradrenaline release.38–40
Therefore, to characterize the implication of dopamine on tactile funnelling illusion (TFI), we evaluated the effects of dexamphetamine (0.45 mg/kg) on temporal and spatial BWs in a larger sample population of healthy participants than previously used for RHI tests. The TFI is a simple and inexpensive tool to test tactile illusions by manipulating spatial and temporal conditions.41,42 Therefore, it is an ideal means to test effects of dexamphetamine on both temporal and spatial BWs in the unimodal somatosensory domain, and was chosen for those purposes. We hypothesized that dexamphetamine would increase the experience of unimodal illusory perception and BWs as it previously affected RHI.
Methods
The University of Western Australia (UWA) Human Research Ethics Committee granted ethical approval for this study (RA/4/1/7557). The study is registered at the Australian New Zealand Clinical Trials Registry (ACTRN12615000619549). All 46 participants were recruited via lecture announcements, word of mouth, advertisements on campus notice boards, social media and university group emails. We sent a prospectus package that contained the participant information form, participant consent form, consumer medication information on dexamphetamine and contact information to those who showed interest. We informed participants that they should abstain from any psychoactive substance 24 hours before the test.
We included participants if they were between the ages of 17 and 60 years, and female participants who were not pregnant and who were using contraceptives to avoid pregnancy if they were sexually active and fertile. We excluded participants if they had heart or severe blood vessel disease; high blood pressure (systolic > 140 mm Hg, diastolic > 90 mm Hg); glaucoma; hyperthyroidism; tics (including Tourette syndrome or a family history of Tourette syndrome); sensitivity to dexamphetamine; any degenerative disease of the nervous system; epilepsy or other neurologic disorders, including head injury; psychiatric or psychological problems; a serious medical problem for which they were receiving or had received treatment; substance abuse disorder; a family history of schizophrenia in their first-degree relatives (parents, children or siblings); use of any drug, including alcohol or any illicit drug, within 24 hours of each testing session; used caffeine on the day of testing; had taken prescription medication other than oral contraceptives or acne medication; or used over-the-counter medication in the 48 hours before each testing session.
Participants
We recruited 46 healthy participants (20 female) with a mean (± standard deviation [SD]) age of 22.9 (± 4.7) years and a mean weight of 71.7 (± 14) kg.
Drug and design
We used a randomized, double-blind, counterbalanced placebo-controlled crossover study in which the participant received either dexamphetamine or placebo, in a counterbalanced permuted block randomization (i.e., randomized in sets of 4 consecutive participants) fashion such that there were equal numbers with placebo first and active drug first. We administered dexamphetamine (0.45 mg/kg, taken orally; Aspen Pharmacare, Australia), giving a mean dosage of 32.3 (± 6.2) mg based on the mean weight of the participants of 71.7 kg. Exact same sizes and numbers of capsules containing either placebo (glucose) or dexamphetamine (0.45 mg/kg) were prepared using 1, 2.5, 5 and 10 mg tablets of dexamphetamine sulfate. We selected this dose based on previous studies in healthy participants that showed significant effects on a range of illusory and psychophysiological measures.22,23,43
The testing session ran from 9 am to 3 pm over a total of 2 days (with a minimum separation of 1 wk) for each participant. The drug or placebo was administered at 9 am, and then physiologic measurements and demographic data were taken. Transportation and lunch were provided with no additional financial incentive. Informed consent was obtained and, in addition to the exclusion criteria, formal medical and psychiatric assessments by psychiatrists were conducted before the experiment on the first day. We then recorded the demographic information (sex, age and weight).
General procedures
Procedure for tactile funnelling illusion
We administered the TFI test immediately after lunch (i.e., lunch was around 200 min after administration of the drug, and TFI was conducted at about 230 min). Participants completed a separate task (the marble hand illusion) between the psychological tests and TFI. We will analyze and report on the data from this other illusory task separately. The participant was seated and then blindfolded. Their dominant arm was placed on the table with the palm facing the ceiling. For the reference point, a straight line was drawn across the arm 5 cm below the elbow. We started the tests of the tactile (touch) using the compass (caliper) at a distance of 5 cm, then at 1 cm reductions to a final spacing of 1 cm (i.e., 5, 4, 3, 2 and 1 cm). There were 5 touches at each distance, and the touch was meant to be light (around 2 mm deep) in areas with less hair. We placed 1 point of the caliber on the reference line and then indented different points on the reference line for 5 touches at each distance (a total of 75 touches per participant per session). There were 3 temporal conditions: 0, 500 and 750 ms (Appendix 1, available at jpn.ca/lookup/doi/10.1503/jpn.220149/tab-related-content). For the 2 asynchronous conditions (500 and 750 ms), the TempoPerfect Metronome software program was used by the examiner as an alarm for the delay between the 2 touches. The order of delays was 750–500–0 ms. We measured funnelling (the number of touches perceived) and error of localization (EL) as outcomes. Funnelling is the awareness aspect of the illusion (if the participant perceived 1 touch instead of 2), whereas EL is the error of spatial localization from the reference line (0 cm).
Psychological scales
We administered the following 3 rating scales once per day (90 min after treatment) during our study: Brief Psychiatric Rating Scale (BPRS),44 Scale for the Assessment of Positive Symptoms (SAPS)45 and Magical Ideation Scale (MIS).46
Physiologic measures
We recorded blood pressure (BP), heart rate and temporal body temperature (temperature) 5 times each day, in triplicate, to follow the time course of drug and placebo effects on autonomic functioning. We recorded BP and heart rate using an Omron HEM-7121 (RML32) automatic BP monitor (Kyoto, Japan), and body temperature (°C) was recorded using a MedeScan RC008 touchless thermometer (Condell Park, Australia). The consecutive physiologic testing times were 0, 60, 110, 210 and 370 minutes after drug administration.
Statistical analysis
We used R version 3.6.3 (R Core Team 2020), and dply, ez, mblm, lme4, plyr and Rmisc packages to perform the statistical analysis. We used repeated-measures analysis of variance (ANOVA) to analyze the data, with delay, distance and drug condition as within-participants factors and drug order as a between-participants factor.
We inspected plots of the residuals and Q–Q plots to ensure the residuals approximated a normal distribution. We then conducted paired t tests for pairwise comparisons between drug and placebo. We used Wilcoxon rank–sum tests with continuity correction if the residuals deviated substantially from normality. Accordingly, we analyzed the TFI of the overall participants using repeated-measures ANOVA with Greenhouse–Geisser corrections if the assumption of sphericity was violated (all degrees of freedom reflect the Greenhouse–Geisser–corrected degrees of freedom where sphericity was violated) and calculated generalized η2 effect sizes. Each psychological scale was analyzed using Wilcoxon rank–sum tests with continuity correction. All pairwise comparisons are with Bonferroni correction. Exceptions to these methods are listed in the Results.
As a common statistical model, we have taken into consideration the combined effect (i.e., interaction) of 2 or more than 2 variables (e.g., delay and distance) on an outcome (i.e., funnelling or EL). Widaman47 and Buckless and Ravenscroft48 suggested that it is preferable to develop statistical methods for testing the relation between dependent and independent variables or for testing ordinal or disordinal interactions. An ordinal interaction has nonsymmetrical patterns of cell means or has “the cross-over of predicted values at the boundary or outside the range of observed values” (e.g., Figure 1A in our case), whereas a disordinal interaction has a symmetric pattern of cell means or has “a cross-over of predicted values within the observed range of values” (e.g., Figure 1C in our case).47,49 In other words, ordinal interaction presents “when the effect of one factor is in the same direction for each level of the other factor while it may vary in effect size.” A disordinal interaction is also called “crossover interaction” or “double dissociation” and presents “if a factor has opposite effects across at least 2 levels of the other factor.”50 Therefore, it has been suggested that researchers should identify or state the detail of whether they predict ordinal or disordinal interactional effects. We have predicted ordinal interactions. Importantly, an increased number of factors or levels of factors may result in unexpected analytical outputs.51 Interaction effects identified using ANOVA are more reliable.52 However, as conventional ANOVA commonly detects main effects and is less powerful for testing ordinal interactions,48,53 planned comparisons are applied to identify whether there are interaction effects or not.51,53
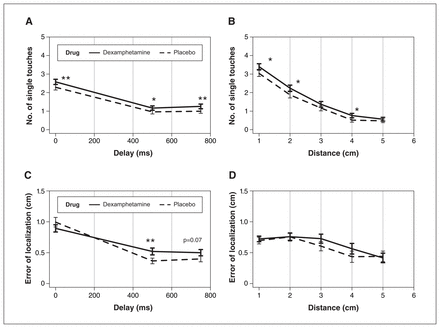
Effects of dexamphetamine (0.45 mg/kg, administered orally) on (A and B) funnelling and (C and D) error of localization (EL) versus (A and C) delay conditions or (B and D) distance conditions. Data are presented as numbers of 1 touch (single touches felt) versus (A) delay or (B) distance for funnelling; EL versus (C) delay or (D) distance. Although there was a significant interaction of only drug and delay on EL across (C) delay, pairwise comparisons indicated that there were significant effects of dexamphetamine on (A and B) funnelling across (A) each delay condition and (B) some distance conditions. ***p < 0.001; **p < 0.01; *p < 0.05.
Power analysis
We used the G*Power 3.1 power calculator and based our predicted mean difference (drug–placebo) and SD of the difference on findings in our laboratory on the effect of dexamphetamine on the “embodiment” component of the RHI, with an effect size of 0.43 (α = 0.5), with a power of 0.80 (80%) with a 2-tailed test.
Results
Tactile funnelling illusion
We analyzed TFI with 2 outcomes: the number of times 2 touches were perceived as 1 (funnelling) and EL away from the reference line. There were significant main effects of dexamphetamine on funneling (F1,45 = 7.3, p = 0.009, η2 = 0.008) but not on EL (F1,45 = 0.65, p = 0.43, η2 = 0.001) (Appendix 1). However, as shown in Figure 1, we found significant interactions between drug and delay on EL (F1.4,63 = 3.7, p = 0.03, η2 = 0.0052) but not on funnelling (F2,90 = 0.14, p = 0.87, η2 = 0.00001). There were also no significant interactions between drug and distance (F4,180 = 0.92, p = 0.45, η2 = 0.0013), and drug, distance and delay (F8,360 = 0.3, p = 0.96, η2 = 0.0007) on funnelling. For EL, we found no significant interactions between drug and distance (F4,180 = 0.92, p = 0.45, η2 = 0.0013) or drug, distance and delay (F8,360 = 1, p = 0.36, η2 = 0.0037).
We also found significant main effects of delay (F1.12,50.4 = 30, p < 0.0001, η2 = 0.29) and distance (F2.1,95.4 = 77.41, p < 0.0001, η2 = 0.33) on funnelling, and a significant effect of delay (F1.3,60 = 25.3, p < 0.00001, η2 = 0.087) and distance on EL (F2.7,124 = 5.45, p = 0.0001, η2 = 0.027), which show that increasing either the delay or distance condition decreases funnelling illusion (Figure 1). Consistent with a spatiotemporal interaction on illusory perception that showed that increasing both delay and distance condition decreases illusion, we found significant interactions between delay and distance on EL (F3,270 = 5.16, p = 0.00001, η2 = 0.03). However, there were no significant interactions between delay and distance on funnelling (F8,360 = 1.4, p = 0.19, η2 = 0.0045).
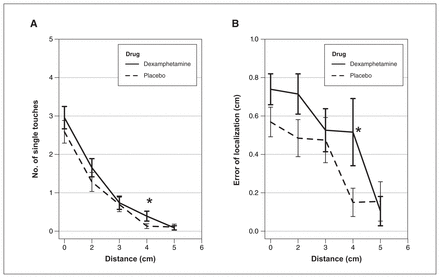
We also found significant drug effects on EL at specific delay (500 ms) and distance (4 cm) conditions, showing that dexamphetamine increases temporal and spatial BWs, respectively (Figure 2).
The effect of dexamphetamine (0.45 mg/kg, administered orally) on (A) funnelling and (B) error of localization (EL) across 500 ms. Data are presented as (A) number of single touches or (B) EL versus distance across 500 ms. Illusion outcomes of dexamphetamine and placebo are significantly different from each other on the funnelling and EL across delay and distance conditions at 500 ms and 4 cm (p < 0.05 for both), although the interaction between delay and drug is significant only for EL.
Psychological measures
Table 1 presents the effect of dexamphetamine and placebo on each psychological scale. The Wilcoxon rank–sum test with continuity correction showed that dexamphetamine significantly increases BPRS (V = 767, p = 0.0000001), MIS (V = 441, p = 0.0008) and SAPS (V = 552, p = 0.00009).
Psychological effects of dexamphetamine and placebo
Physiologic measures
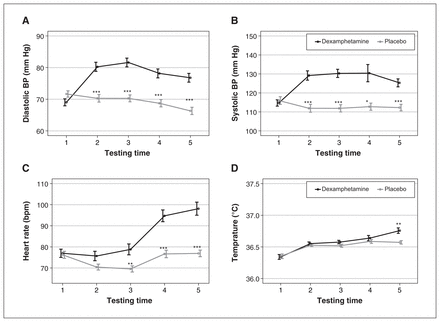
Physiologic data from 1 participant were missing, leaving data for 45 participants for our analysis. Analysis of variance showed that there were significant main effects of dexamphetamine on diastolic BP (F1,44 = 122.6, p < 0.0001, η2 = 0.18), systolic BP (F1,44 = 78.02, p < 0.0001, η2 = 0.15), temperature (F1,44 = 4.7, p = 0.03, η2 = 0.02) and heart rate (F1,44 = 48.14, p < 0.0001, η2 = 0.13). There were significant interactions between drug and time on diastolic BP (F3.2,140.8 = 22.6, p < 0.0001, η2 = 0.09), systolic BP (F2,88 = 4.72, p = 0.00018, η2 = 0.05), temperature (F3.1,132.7 = 3.3, p = 0.0036, η2 = 0.023) and heart rate (F3.3,144.3 = 29.4, p < 0.0001, η2 = 0.14) (Figure 3).
The effect of dexamphetamine (0.45 mg/kg, administered orally) on (A) diastolic blood pressure (BP), (B) systolic BP, (C) heart rate and (D) body temperature as a function of time. Relative to the placebo, dexamphetamine significantly raised both (A) diastolic BP and (B) systolic BP at the 4 testing times after drug administration (2–5). Dexamphetamine significantly raised (C) heart rate and (D) temperature at 3 (3–5) and 1 (5) testing times after administration, respectively. The peak effect of dexamphetamine on diastolic BP was at measurement time 3, whereas it was at times 3 and 4 for systolic BP. *p < 0.05; **p < 0.01; ***p < 0.001. The 5 testing times were 0, 60, 110, 210 and 370 minutes after drug administration.
Discussion
We investigated the role of dopamine or catecholamines on tactile BWs by administering dexamphetamine (0.45 mg/kg) to healthy participants undergoing the TFI. Our findings were as follows: dexamphetamine increased the funnelling (the awareness aspect of the illusion) strength, but the results failed to show evidence of widened delay or spatial BWs for funnelling because there were no temporal or spatial condition–dependent changes in the funnelling illusion relative to placebo; dexamphetamine increased the EL at the 500 ms asynchronous delay condition, which indicated widened temporal BWs; and dexamphetamine increased EL illusion specifically at 4 cm, which indicated widened spatial BWs. Overall, our findings show that dexamphetamine widens spatial and temporal BWs. The effect of dexamphetamine on BWs in the TFI in our study is in agreement with the increase in the visuo-tactile RHI test in healthy participants after receiving dexamphetamine that was reported in a 2011 study,22 and in projected hand illusion (PHI) or RHI tests that showed people with schizophrenia experienced increased illusion under asynchronous delay conditions.13,21,54–56
A 2011 double-blind, placebo-controlled crossover study showed that dexamphetamine increased embodiment during the RHI for both synchronous and asynchronous stimulation in healthy participants.22 Like this RHI study,22 we found an increase in temporal BWs after administration of dexamphetamine. We also found that dexamphetamine increased spatial BWs in the TFI. Our findings and those of previous studies in schizophrenia21,54 or that used dopaminergic agents in healthy participants22 that reported widening of temporal BWs on multimodal illusions may be linked to increased dopamine.
Various illusion tests have shown that people with schizophrenia experience more illusion in PHI,12–14 in RHI,21,54 and in auditory, visual or audiovisual55 than healthy controls. For instance, Graham and colleagues56 reported that asynchronous stroking of the hand at 500 ms in people with schizophrenia produced more illusion in PHI. This supports the idea that dexamphetamine-induced increases in dopamine transmission are representative of results expected to be observed in people with schizophrenia.
However, the exact role of dopamine in illusory perception and BWs is poorly understood but may involve modulating activity of the temporoparietal junction. The merging of stimuli into a perception of a unified object or event has been proposed to result from activity in the temporoparietal junction.57–59 The mesocorticolimbic pathway may modulate activity in the temporoparietal junction via dopamine release in the frontal cortex, which has connections with the temporoparietal junction.60 Importantly, a 2013 review of positron emission tomography and single-photon-emission computed tomography studies clearly showed that dopamine influences psychosis through mesocorticolimbic and nigrostriatal dopaminergic pathways.61 Some studies that reviewed spatial learning showed that dopaminergic projections from these circuits influence spatial information (such as sensory or proprioceptive) in the hippocampus.62,63 It has also been suggested that the somatosensory receptive field in the cortex might be responsible for binding of tactile information,64 which is also influenced by temporal and spatial variations.65 These cortex areas might also be a target area for the dopaminergic influence of the tactile BWs.
Animal studies have shown that cortical66,67 and subcortical areas 68–70 are responsible for processing the cortical binding of tactile stimuli presented at similar or different modalities on different skin sites. For example, during synchronous stimulation of fingers, the recorded cerebral potential was less than the simple addition of the potential produced after stimulation of individual fingers.64,69
We further found that dexamphetamine increased the funnelling effect, although it failed to expand BWs for the funnelling effect. Previous studies have suggested that “afferent-induced inhibition” causes funnelling illusion,41,71,72 which may show that dexamphetamine strengthens the afferent-induced inhibition process in the tactile neuron.68,69 The effect of dexamphetamine on the BWs of EL (without affecting the BWs of funnelling) may indicate that the spatial localization of the single touches felt are dependent more on the internal body schema or map than is the funnelling (awareness of the number of touches) in the absence of visual cues. This further shows that the conscious awareness of sensory processing (e.g., touch perception) is not well mapped into the unconscious body schema (superficial schema or body form representation), which depends on somatosensory and visual inputs, and is involved with tracking and predicting body parts that allows localization of tactile perception.73–75
Further research should be done to explore the relation between dopamine or catecholamines and BWs. Our finding may explain that the increased amount of catecholamines by dexamphetamine is sufficient to increase the experience of the TFI and increase BWs. These results also show that the TFI is sensitive to both temporal and spatial conditions that can be further influenced by drugs. The spatial limits showed a nonlinear relation in the strength of EL, which was similar to a previous study that applied 6 spatial positions (17.5–67.5 cm) during RHI tests and reported that a significant nonlinear relation in the strength of the illusion.76 It has been shown that RHI is sensitive to spatial domain (match/mismatch)77 and temporal condition.78 Albrecht and colleagues22 reported that dexamphetamine increased the embodiment score (mainly the ownership one) during both synchronous and asynchronous conditions. In a self-face recognition test, enfacement illusion was also strong under synchronous delay conditions.78–80 Similar to our study, Kahrimanovic and colleagues65 reported that synchronous stimulation of fingers during a tactile experiment caused assimilation effect, whereby there was a high probability of integrating 2 or more simulations as 1. For the TFI, the upper limit of the temporal BWs was reached at the 500 ms delay condition; we suggest that future research target shorter delay conditions to identify the lower bounds of the window. Finally, the present effect of dexamphetamine on spatial and temporal BWs may add to the existing dopamine hypothesis of schizophrenia, although the important drawbacks of using d-amphetamine precludes the degree of association. The disadvantages of the d-amphetamine model of schizophrenia are the complex nature of schizophrenia that could not be mimicked or explained by psychosis induced by d-amphetamine and the nonselective release of monoamines after d-amphetamine administration.25,26,30,31,81,82
The important strengths of our study were its design and use of the temporal and spatial conditions to evaluate BWs. We recruited 46 participants, more than double the number of participants in previous dexamphetamine studies on BWs.22,83 Importantly, our previous TFI study involving 20 healthy participants showed that, although dexamphetamine influenced funnelling illusion based on changes in psychometric score, it failed to modify BWs.83
We manipulated and compared the illusory and psychological effects of dexamphetamine at a moderate dose (about 33 mg), which was 2–3 times higher than that used in most previous dexamphetamine challenge studies. We involved participants whose ages and education levels were very close, which provided a homogeneous population and avoided or decreased age-related cognitive differences.84–88
There are, however, some important limitations to be noted. We did not measure plasma levels of dexamphetamine to correlate the plasma concentration of dexamphetamine with BWs. However, we conducted the TFI measurement within about 30 minutes of the expected peak concentration of dexamphetamine (i.e., the study was conducted at 230 min). Previous studies have shown that peak plasma concentrations of 0.4–0.5 mg/kg after dexamphetamine administration occur around 180–240 minutes89–91 and decrease to 75% of the maximum level after 500 minutes.89 Asghar and colleagues89 and Silber and colleagues92 found that the psychological, physiologic or cognitive performance changes did not predict the time course of plasma levels of dexamphetamine. In addition, the half-life of dexamphetamine is about 12 hours, which allows the drug to be active during all psychological or cognitive measurements as shown by the physiologic measurements conducted before and after the TFI. Another limitation is related to the neurochemical model and interpretation. Dexamphetamine induces the release of other neurotransmitters such as noradrenaline93 and, to a much lesser extent, serotonin,94 both of which may affect BWs. Therefore, the inferences drawn from our findings may be confounded because noradrenaline pathways or the interaction between dopaminergic and noradrenergic pathways could be an alternative explanation for the effects of dexamphetamine.26,30,82 Further studies could use selective dopaminergic agents to isolate the effects of dopamine or noradrenaline on BWs. We did not verify abstinence from other psychoactive substances by urine screening, although a previous validation study in our laboratory showed that self-reports regarding recent substance use (e.g., cannabis, within 24 h) were consistent and valid (κ = 0.91).95
Conclusion
We have shown that increasing catecholamine release through the administration of dexamphetamine (0.45 mg/kg) increases temporal and spatial BWs in the unimodal funnelling illusion. Further studies using selective dopaminergic and noradrenergic agents will be needed to elucidate whether the observed dexamphetamine effects are driven by dopamine release specifically, as dexamphetamine releases catecholamine.
Acknowledgment
The authors thank all volunteers for their participation, and all colleagues who assisted in the data collection.
Footnotes
Competing interests: Mathew Martin-Iverson has received payments for expert testimony from the courts of Australia. No other competing interests were declared.
Clinical trials registry: ACTRN12615000619549
Funding: This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
- Received August 24, 2022.
- Revision received September 28, 2022.
- Revision received October 25, 2022.
- Revision received November 9, 2022.
- Accepted November 11, 2022.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/