A 41-year-old unemployed woman with longstanding major depressive and social anxiety disorder was referred to the Metabolic Clinic at the Centre for Addiction and Mental Health for weight gain concerns. She had maintained a weight of about 54 kg during adulthood, until she gained 41 kg over a period of 1.5 years during the course of several antipsychotic and antidepressant trials. The patient’s current psychotropics and metabolically active medications included quetiapine (300 mg/d at bedtime), ketamine (250 mg, inhaled every 3 days), zopiclone (22.5 mg/d at bedtime), baclofen (55 mg/d), lorazepam (2 mg at bedtime) and sumatriptan (100 mg/d as needed). She preferred to continue quetiapine given the stability of her psychiatric symptoms. Her psychotropic medications were managed by her treating psychiatrist.
The patient weighed 95.6 kg at the time of consultation, translating to class III obesity and a body mass index (BMI) of 42.5 kg/m2. She met criteria for abdominal obesity, with a waist circumference of 108 cm. Bloodwork showed evidence of metabolic dysfunction, with elevated levels of fasting glucose (6.4 mmol/L), fasting insulin (297 pmol/L), low-density lipoprotein (LDL) cholesterol (2.93 mmol/L), total cholesterol (5.87 mmol/L) and triglycerides (2.35 mmol/L). High-density lipoprotein (HDL) cholesterol was 1.87 mmol/L. We recommended metformin, a well-tolerated antihyperglycemic agent that has the strongest evidence of benefit for antipsychotic-induced weight gain.1 We initiated 500 mg/d and titrated the dose to 2500 mg/d. The patient concurrently implemented lifestyle modifications, including improved diet and increased physical activity, walking up to 5 times per week for up to 2 hours each time.
With metformin and the lifestyle changes combined, the patient lost about 45 kg over 3 years. No adverse effects were reported, and her weight loss was gradual at 1–3 kg per month (Table 1). She weighed 49.2 kg at her most recent visit, and both her BMI and waist circumference normalized to 21.9 kg/m2 and 65 cm, respectively. Improvements in other metabolic parameters were observed, including reduced triglycerides (down to 1.26 mmol/L), fasting glucose (5.6 mmol/L), and insulin (61 pmol/L) levels. Her mood symptoms and her psychotropics remained unchanged through this period.
Summary of change over 3 years of treatment with metformin
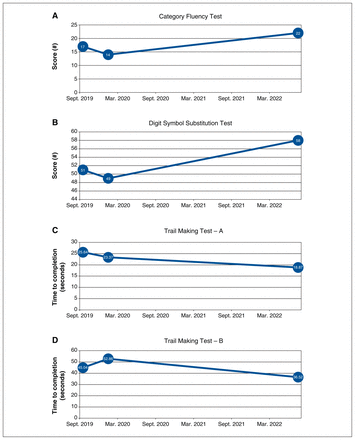
Interestingly, the patient showed improvements in cognitive functioning over this period (Figure 1). In keeping with a measurement-based care approach, we examined semantic memory using a Category Fluency Test (CFT), attention and processing speed using the Digit Symbol Substitution Test (DSST) and the Trail Making Test A (TMT-A), and executive functioning using the Trail Making Test B (TMT-B) at baseline and after 3 years. She had a 5- and 7-point increase on the CFT and DSST, respectively, which has been shown to be clinically meaningful. 2 Her completion time for the TMT-A and TMT-B also improved by 6.77 seconds and 8.52 seconds, respectively. She reported subjective improvements in attention, allowing her to re-engage in crocheting, a mentally stimulating task involving several cognitive domains.
Trajectory of cognitive test scores over a 3-year interval (testing occurred in September 2019, January 2020 and July 2022).
Antipsychotics are increasingly prescribed for nonpsychotic disorders. 3,4 They have well-established efficacy in reducing psychiatric symptoms5 and improving functionality6; however, the cardiometabolic risks of these medications are increasingly recognized.
Metabolic derangements occur rapidly within months of their use and progress over time.7 Within 3 months of antipsychotic initiation, people experience a mean weight gain of 3.22 kg,8 and by 6 months almost half develop overweight or obesity. 9 One in 5 patients treated with antipsychotics develop hyperglycemia, 10 of whom half progress to diabetes.11 These metabolic derangements are associated with impaired quality of life12 and possibly with cognitive decline.13 Indeed, people with severe psychiatric disorders and comorbid metabolic syndrome show worse cognitive functioning than those without metabolic comorbidity. 14–16 In the general population, metabolic dysfunction is associated with deficits in memory, visuospatial abilities, executive functioning and processing speed as well as an increased risk for dementia.13,17,18
Emerging evidence suggests that improving metabolic dysfunction may improve cognitive functioning. Both surgical19 and pharmacological20 weight loss interventions have been associated with improved cognition. Our patient showed improved cognitive performance that paralleled the resolution of her metabolic dysfunction, suggesting a possible relation.
Various mechanisms have been postulated to underlie this association, including neuroinflammation, oxidative stress, abnormal brain lipid metabolism, brain insulin resistance and reduced vascular reactivity.17,21 Through these pathways, interventions targeting metabolic dysfunction may confer benefits beyond physical health. In our patient’s case, metformin could have improved cognitive functioning by reducing central and peripheral inflammation,22,23 and/or by increasing peripheral and brain insulin sensitivity.23,24 Emerging evidence suggests that brain insulin resistance may underlie the bidirectional association between cognitive and metabolic disorders.25
Given the association between metabolic health and cognition, clinicians should consider early metabolic interventions to dually improve physical health outcomes and safeguard against cognitive decline in patients with comorbid psychiatric disorders and obesity. While several options are available, we suggest starting off-label metformin as the first line of intervention along with diet and lifestyle changes in patients with antipsychotic-induced weight gain or metabolic dysfunction whose psychiatric stability may depend on antipsychotic continuation. See Agarwal and colleagues26 for a practical guide on using metformin in psychiatry. Moreover, it is imperative to measure cognition in studies that examine the effects of metabolic interventions in obesity. Further research is needed to elucidate the mechanisms connecting metabolic and brain health.
Acknowledgements
N. Stogios is supported by the Ontario Graduate Scholarship and the Banting and Best Diabetes Centre (BBDC) Novo Nordisk Graduate Studentship. M.K. Hahn is supported in part by an Academic Scholars Award from the Department of Psychiatry, University of Toronto; has grant support from the BBDC, the Canadian Institutes of Health Research (CIHR; PJT-153262) and the PSI Foundation; and holds the Kelly and Michael Meighen Chair in Psychosis Prevention, and the Cardy Schizophrenia Research Chair. S.M. Agarwal is supported in part by an Academic Scholars Award from the Department of Psychiatry, University of Toronto and has grant support from the CIHR and the PSI Foundation.
Footnotes
↵* Share first authorship.
The information in this column is not intended as a definitive treatment strategy but as a suggested approach for clinicians treating patients with similar histories. Individual cases may vary and should be evaluated carefully before treatment is provided. The patient described in this column gave informed consent for its publication.
Competing interests: None declared.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/