Abstract
Background: Factors affecting the effectiveness of electroencephalogram-based neurofeedback (EEG-NF) against the core symptoms of attention-deficit/hyperactivity disorder (ADHD) remain unclear.
Methods: We searched the PubMed, Embase, Web of Science, ClinicalKey, Cochrane CENTRAL, ScienceDirect, and ClinicalTrials.gov databases from inception to August 2022 for randomized controlled trials (RCTs) on patients with ADHD involving outcome assessments of improvements on behavioural rating scales of inattention, hyperactivity/impulsivity, and global symptoms. Comparators included nonactive (e.g., wait list/treatment as usual) and active (e.g., cognitive training) controls.
Results: Our analyses included 21 RCTs comprising 1261 participants. Our results demonstrated significantly better improvement in symptoms of inattention, hyperactivity/impulsivity and global symptoms of ADHD associated with EEG-NF than comparators from both proximal (p = 0.01, p = 0.02 and p = 0.01, respectively; e.g., parents) and distal (p = 0.01, p < 0.05 and p = 0.01; e.g., teachers) raters. Meta-regression revealed a positive association between therapeutic effects of EEG-NF and intelligence quotient (IQ) from observations of the most proximal raters. Subgroup analysis for studies combining 2 EEG-NF protocols showed better therapeutic effectiveness against symptoms of ADHD than those using a single NF protocol, whereas subgroup analysis adopting a double-blind design failed to demonstrate superiority of EEG-NF to sham control. Moreover, therapeutic effectiveness of EEG-NF was significantly better when wait list/treatment as usual comparators were used compared with sham/placebo EEG-NF controls on subgroup analysis.
Limitations: Our findings are limited by the lack of a double-blind design in most of the studies included in our analyses.
Conclusion: Our results support the effectiveness of EEG-NF for improving inattention, hyperactivity/impulsivity, and global symptoms in patients with ADHD. The high risk of detection and performance bias warrants further study.
Introduction
Electroencephalogram-based neurofeedback (EEG-NF) is a form of biofeedback involving real-time visual or audio feedback based on EEG signals from sensors on the participant’s scalp to reinforce normal brain function via operant conditioning. 1 It has been widely used as an alternative or complementary option in the enhancement of motor learning in patients after strokes,2 and reinforcement of neurocognitive functioning in those with epilepsy,3 as well as a myriad of other neurologic or psychiatric conditions.1
Attention-deficit/hyperactivity disorder (ADHD), a behavioural and neurodevelopmental disorder, is characterized by the core symptoms of inattention, hyperactivity and impulsivity. 4 The rationale for using EEG-NF for patients with ADHD lies in the observation of specific patterns of brain EEG activities in these patients,5 such as elevation in relative θ power, reduction in relative α and β activities, and elevated θ:α and θ:β ratios.6 To target the core symptoms of ADHD, there are 2 main EEG-NF protocols: the modulation of the θ:β (4–7 Hz:12–21 Hz) ratio (TBR) and the self-regulation of slow cortical potential (SCP).5
Although there were a number of important reviews or meta-analyses investigating the effectiveness of this therapeutic approach for improving the core symptoms of ADHD,7–12 the results were mixed. In particular, marked differences in the perception of therapeutic effectiveness of EEG-NF for ADHD were evident between different evaluators (e.g., parents v. teachers),7,8 possibly owing to bias in the assessment process, including evaluators’ bias7 and detection bias when raters were not blinded to the allocated treatment. 13 In addition, although several randomized controlled trials (RCTs) using a double-blind design failed to demonstrate better therapeutic efficacy of surface EEG-NF against symptoms of ADHD than sham controls,14,15 none of the previous meta-analyses addressed these issues.7–10 Moreover, a previous observational study identified an inverse correlation between intelligence quotient (IQ) and the magnitude of information flow,16 implying a potential effect of IQ on brainwave-based intervention approaches, but the possible influence of an individual’s intellectual ability was not investigated in previous meta-analyses.7–10 Finally, despite the clinical use of different EEG-NF protocols to target a variety of brainwave patterns commonly observed in patients with ADHD,5 previous meta-analyses did not focus on the symptom profile (i.e., inattention, hyperactivity and impulsivity) of individuals who would benefit most from specific protocols.
Therefore, through a systematic review of the available RCTs, the present meta-analysis aimed to assess the effects of different factors on the therapeutic efficacy of EEG-NF against the core symptoms of ADHD.
Methods
Electronic searches and registration
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Appendix 1, Table 1, available at www.jpn.ca/lookup/doi/10.1503/jpn.220125/tab-related-content),17 we systematically searched for RCTs using relevant keywords — “neurofeedback” AND “attention or attention-deficit/hyperactivity disorder or ADHD” — in PubMed, Embase, Web of Science, ClinicalKey, Cochrane CENTRAL, ScienceDirect, and ClinicalTrials.gov databases from inception to August 2022. The present study was registered with the International prospective register of systematic reviews (PROSPERO CRD 42021288024). The keywords for literature search using different databases are listed in Appendix 1, Table 2.
Study eligibility and definitions
The eligible criteria were as follows: RCTs on patients diagnosed with ADHD based on the diagnostic criteria in the Diagnostic and Statistical Manual of Mental Disorders (DSM) or the International Classification of Diseases (ICD) systems, or by physicians, or through the use of any standardized rating scales; EEG-NF with comparators including both nonactive (e.g., wait list/treatment as usual) and active (e.g., cognitive training) controls; and outcome assessments with standardized behavioural rating scales from external observation for global or core symptoms of ADHD, such as inattention or hyperactivity/impulsivity. Studies that used only pharmacological interventions in comparison groups or that adopted only self-rating scales for outcome measurements were excluded. There was no restriction on the age of participants, subtypes of ADHD or types of NF protocols adopted in the included studies.
Data extraction
Two independent reviewers (Y.-S.C. and P.-Y.Y.) completed the title, abstract, and full-text screening. We used the κ coefficient to assess interrater reliability.18 Eligibility of the studies for inclusion was determined by 2 reviewers (Y.-S.C. and P.-Y.Y.). Disagreements were resolved through consensus. In case of missing data from the eligible studies, we tried to contact the corresponding authors electronically to obtain the original data. On encountering duplicated data, the latest published article or the article with the largest sample size was selected for analysis.
To examine the validity of the eligible studies, we used criteria developed by the Cochrane Collaboration to assess the risk and possible sources of bias.19
Data synthesis and sensitivity analysis
Therapeutic effects (i.e., pre/post changes) of EEG-NF, assessed using a variety of behavioural ratings scales from observation of the most proximal raters (e.g., parents) or distal raters (e.g., teachers), were quantitatively expressed as effect size (ES) based on Hedges g. For the present study, the most proximal raters referred to those who were closest to the participants and therefore were likely more aware of treatment allocation, whereas distal raters were defined as those who were less close to the participants and therefore were more likely to be blinded to treatment allocation. Although the most proximal raters were parents and the distal raters were teachers in most of our included studies, the assignment of raters as most proximal or distal was based on reviewer consensus (Y.-S.C. and P.-Y.Y.). The behavioural ratings mainly involved inattention, hyperactivity/impulsivity, or global symptoms of ADHD. Effect size was calculated using the computer program Comprehensive Meta-Analysis version for Windows (CMA) version 3.0. Better therapeutic effects in participants receiving EEG-NF than those from comparison groups were indicated as positive values for the calculated ES. The results were standardized and averaged to produce a single ES when a study provided more than 1 data set. We used a random-effects model to evaluate the ES, assuming that the true ES were the same in all eligible studies.20,21
Based on a random-effects model, subgroup analyses were conducted.20 For meta-regression analysis of the correlation between the therapeutic effect and continuous variables (e.g., age, the female percentage, IQ), we used a mixed-effects model. We calculated Q statistics and acquired the corresponding p values for assessing the statistical significance (defined as p < 0.05) of heterogeneity in ES.
Publication bias was assessed with funnel plot inspection when there were fewer than 10 data sets,22 while Egger tests were performed when there were 10 or more data sets.23 On encountering funnel plot asymmetry, potentially missing studies were imputed using the Duval and Tweedie trim and fill method.24 Furthermore, a sensitivity test was conducted using the leave-one-out approach to estimate the effect of each study on the overall ES.22
Results
Study characteristics
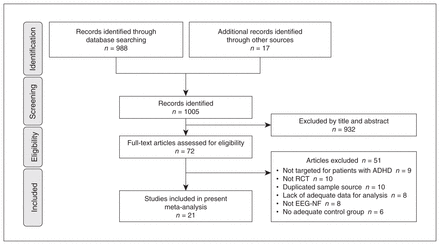
Of the 72 full-text articles initially retrieved, 51 were excluded (Figure 1). Details on the reasons for exclusion are provided in Appendix 1, Table 3. We deemed 21 RCTs comprising a total of 1261 participants to be eligible for inclusion in the present study (Table 1).14,15,25–43 The κ coefficient was 1.0. Comparison groups included treatment as usual, wait list, electromyography (EMG)-biofeedback, computerized attention training, cognitive therapies, behavioural interventions, physical activities and sham/placebo feedback. With regard to treatment protocols, most studies used TBR, 5 studies used SCP training and 3 used combination approaches (i.e., SCP plus TBR). None of the studies included participants with intellectual disability. A variety of behavioural rating scales were used in the included studies (Table 1), and results of these behavioural rating scales were categorized as inattention, hyperactivity/impulsivity, or global symptoms.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram of identifying eligible studies. ADHD = attention-deficit/hyperactivity disorder; EEG-NF = electroencephalogram-based neurofeedback; RCT = randomized controlled trial.
Characteristics of included studies
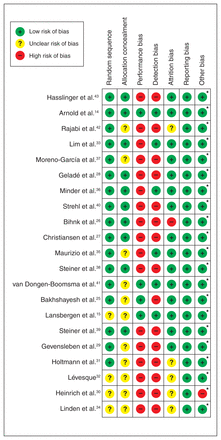
Focusing on the risk of bias, most studies had poor quality regarding performance and detection bias. We found that 63.3 % (93 of 147), 23.1 % (34 of 147), and 13.6 % (20 of 147) of the included studies showed an overall low, high, and unclear risk of bias, respectively. None of the included studies received financial support from pharmaceutical companies (Figure 2).
Risks of bias assessment of the included studies. * Neither the studies nor individual authors of the studies received financial support from pharmaceutical companies.
Quantitative data synthesis
Inattention
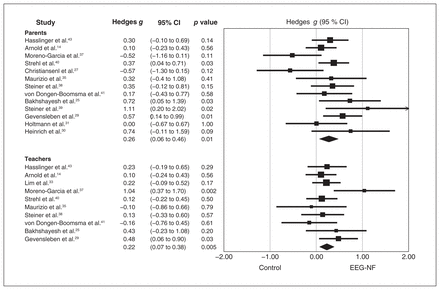
Focusing on the symptoms of inattention, there were a total of 19 studies with available outcomes assessed by most proximal raters (n = 1191, mean age 10.21 [range 8.40–16.14] yr, female gender 19.64 % [range 0%–35.06%]). Of the 19 studies, 14 also provided the outcomes from distal raters (n = 1069, mean age 9.86 [range 8.40–12.40] yr, female gender 22.63% [range 12%–47.80%]) (Table 1). Regarding treatment sessions, the number of sessions varied from 20 to 40, with averages of 31.95 and 31.40 sessions in studies using observations from most proximal and distal raters, respectively. Most studies allowed the use of medications (8%–79.4%) and participants in only 8 studies refrained from undergoing any pharmacological treatment for controlling ADHD symptoms.
Observation data from most proximal raters showed that EEG-NF was significantly superior to comparators in improving inattention among participants with ADHD (Figure 3). The ES was strong in the leave-one-out sensitivity analysis (p = 0.01), suggesting that no single study substantially influenced the overall ES. The Egger test was nonsignificant (p = 0.77), indicating a low risk of publication bias. The observation data from distal raters also showed a significant improvement in participants with ADHD receiving EEG-NF compared with comparison groups (Figure 3). The ES was strong in the leave-one-out sensitivity analysis (p = 0.01), implying that no single study had a significant influence on the overall ES. The Egger test was nonsignificant (p = 0.83), indicating a low risk of publication bias.
Effect sizes for comparing the difference in the improvement of inattention between electroencephalogram-based neurofeedback (EEG-NF) and control comparators based on the observations of most proximal raters (parents) and distal raters (teachers). CI = confidence interval.
Subgroup analysis of the effect of choosing comparators demonstrated a significantly better therapeutic effect of EEG-NF (i.e., a larger ES) against inattention in studies using wait list/treatment as usual than that in those adopting sham/placebo EEG-NF as comparison groups (Table 2). Regarding the influence of different NF protocols, subgroup analysis showed a significantly larger ES in studies combining 2 protocols for improving inattention than that in those using a single NF protocol (i.e., SCP and TBR) (Table 2). Meta-regression showed a significant positive association of the therapeutic effects of EEG-NF against the symptoms of inattention with IQ from observations of most proximal raters (p = 0.01) (Table 3).
Subgroup analysis of factors affecting therapeutic benefits of NF for symptoms of inattention, hyperactivity/impulsivity, and global symptoms in individuals with ADHD (Hedges g)
Regression coefficients of correlations between continuous variables and improvement in inattention, hyperactivity/impulsivity, and global symptoms in included studies using a mixed-effects model
Hyperactivity/impulsivity
Regarding the therapeutic effects of EEG-NF against the symptoms of hyperactivity/impulsivity, 16 studies using evaluation from most proximal observers were identified (n = 952, mean age 10.00 [range 8.40–12.40] yr, female gender 21.09% [range 0%–47.80%]). Additionally, 13 studies using assessment from distal observers were identified (n = 846, mean age 9.95 [range 8.40–12.40] yr, female gender 23.20% [range 12%–47.80%]). We found a significantly better improvement in the symptoms of hyperactivity/impulsivity in participants receiving EEG-NF than in their comparators (Figure 4). Sensitivity analysis with the leave-one-out approach showed no substantial influence of any single study on the overall ES. The Egger test was nonsignificant (p = 0.28 and p = 0.41 for most proximal and distal raters, respectively), suggesting a low risk of publication bias.
Effect sizes for comparing the difference in the improvement of hyperactivity/impulsivity between electroencephalogram-based neurofeedback (EEG-NF) and control comparators based on the observations of most proximal raters (parents) and distal raters (teachers). CI = confidence interval.
Our subgroup analysis focusing on results from most proximal raters showed a significantly larger ES in studies combining 2 protocols on improving hyperactivity/impulsivity than that in those using a single NF approach (i.e., SCP and TBR). In addition, the ES of therapeutic effects of EEG-NF against hyperactivity/impulsivity was significantly larger in studies using wait list/treatment as usual than in those adopting sham/placebo as comparison groups (Table 2). On the other hand, the aforementioned differences in subgroup comparisons were not statistically significant when analyzing results from distal raters (Table 2). Consistently, meta-regression of data derived from the observations of most proximal raters showed that the therapeutic effect of EEG-NF against the symptoms of hyperactivity/impulsivity was positively associated with IQ (p = 0.04) but negatively associated with the proportion of female participants (p = 0.03) (Table 3).
Global symptoms
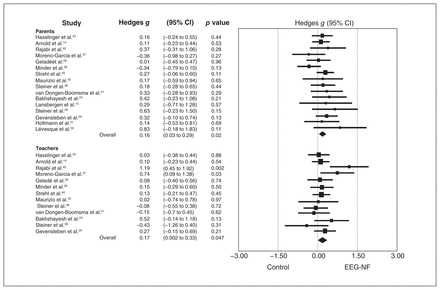
With regard to the therapeutic effects of EEG-NF against the global symptoms of ADHD, there were 13 studies using the evaluation from most proximal raters (n = 691, mean age 9.87 [range 8.40–12.40] yr, female gender 20.90% [range 4.50%–47.80%]). In addition, 12 studies based on the observations from distal raters were noted (n = 874, mean age 9.65 [range 8.40–12.40] yr, female gender 22.81% [range 12%–47.80%]).
The therapeutic effect of EEG-NF was significantly superior to that of compartors against the global symptoms of ADHD from both most proximal and distal raters (Figure 5). Sensitivity analysis showed that no single study had a significant impact on the overall ESs. The Egger test was nonsignificant (p = 0.93 and p = 0.52 for most proximal raters and distal raters, respectively), indicating a low risk of publication bias.
Effect sizes for comparing the difference in the improvement of global symptoms between electroencephalogram-based neurofeedback (EEG-NF) and control comparators based on the observations of most proximal raters (parents) and distal raters (teachers). CI = confidence interval.
Our subgroup analyses showed a significantly larger ES in the therapeutic effect of EEG-NF against the global symptoms of ADHD in studies combining 2 NF strategies (i.e., TBR plus SCP) than in those using a single NF protocol based on observations from both most proximal and distal raters, whereas a better therapeutic effect (i.e., larger ES) of EEG-NF against the global symptoms of ADHD in studies adopting wait list/treatment as usual compared with those using sham/placebo EEG-NF as comparators was noted only in observations from most proximal raters (Table 2). Meta-regression analysis did not show any significant association between the therapeutic effect of EEG-NF against the global symptoms of ADHD and the investigated continuous variables (e.g., age, prevalence of females, number of sessions, medications, global overall symptom, and follow-up duration) (Table 3).
Discussion
In comparison with several important reviews and meta-analyses investigating the therapeutic effects of EEG-NF against the core symptoms of ADHD,7–11 our meta-analysis was more comprehensive and updated. In particular, while some meta-analyses attempted to conduct separate analyses to compare trials using active (e.g., cognitive training) and nonactive (e.g., wait list/treatment as usual) controls, none of them conducted subgroup analyses on RCTs using a double-blind design. Moreover, none of the previous meta-analyses performed meta-regression to investigate the possible influences of other factors, such as IQ, on the efficacy of EEG-NF. Our study showed that adoption of a double-blind design and that certain factors, such as IQ, could affect the therapeutic benefits of EEG-NF. Finally, by including more recent studies that showed a lower risk of allocation, performance and detection biases, our study may provide more reliable results, as reflected by similar ES between most proximal and distal raters.
An interesting finding from previous studies was the higher therapeutic efficacy of EEG-NF (i.e., larger ES) based on the observations of most proximal raters (usually parents) than that from distal raters (mostly teachers), indicating the probability of detection bias.7,8 Nevertheless, an assumption that parents compared with teachers are always in favour of the therapeutic effects of EEG-NF may not reflect the actual scenario, because many trials collected data only from parents.7,8 In particular, the study by Lévesque and colleagues showing a large ES for therapeutic efficacy of EEG-NF did not provide data from teachers’ observations.32 Therefore, despite the lower risk of detection bias from teachers’ observations (i.e., probably blind) compared with that from parents, there may be other factors leading to discrepancies in the perception of therapeutic effects of EEG-NF among different observers.
Our subgroup analyses further suggested that differences between observations from most proximal and distal raters may be associated with the choice of control groups in the included studies. Our subgroup analyses showed a large discrepancy in the perception of therapeutic effects of EEG-NF between most proximal and distal raters in studies using wait list/treatment as usual as control groups (i.e., ES for symptoms of inattention 0.51 v. 0.39 from proximal and distal raters, respectively), whereas this difference was much smaller in trials using sham/placebo feedback for controls (ES for symptoms of inattention −0.03 v. 0.08 from proximal raters and distal raters, respectively). The disappearance in the present study of discrepancies between most proximal and distal observers when both were blinded to treatment allocation may suggest the presence of detection bias.
Although detection bias may be a confounder if observers are not blinded to the allocated treatment,13 the present study showed that EEG-NF was still more effective than nonactive treatment for symptoms of inattention in our subgroup analysis from observations of distal raters despite the smaller ES than that from most proximal observers. In addition to the possible improvement in attention by providing NF to train individuals to correct abnormal brainwave patterns,1 there are other factors that may lead to the observed therapeutic effects of surface EEG-NF. For instance, the Hawthorne effect may be a contributor, as confidence in technology and fascination with brain science may further enhance one’s motivation for learning.44 This is supported by our subgroup analysis involving only studies with a double-blind design, demonstrating that surface EEG-NF was no longer superior to sham/placebo feedback treatment from both most proximal and distal observations. However, such an apparent lack of beneficial impact of EEG-NF needs to be interpreted with caution because all sham conditions involved some behavioural therapeutic elements and all 3 studies concluded that both NF and sham control were effective for improving symptoms of ADHD.15,41,45 Nevertheless, from the results of our subgroup analyses, current evidence is still insufficient to support an additional therapeutic effect of NF against the symptoms of ADHD. In fact, some studies failed to show an association between improvement in behaviours and correction of underlying brainwave patterns.46,47 Further studies with a double-blind design are necessary to confirm the benefit of providing NF in addition to concurrent cognitive behavioural therapies.
Moreover, despite the positive therapeutic effects of EEG-NF against the symptoms of ADHD from both most proximal and distal observers regardless of the choice of comparators in the present study, the therapeutic benefit was evident only in the majority of studies adopting wait list/treatment as usual as controls, but not in those using sham controls. Taking into account our finding of a significant difference in the therapeutic effectiveness of EEG-NF between subgroups of studies adopting wait list/treatment as usual and those using sham controls as comparators (i.e., inattention, hyperactivity/impulsivity, and global symptoms from most proximal observers; symptoms of inattention from distal observers), the overall effectiveness of EEG-NF may be due to the larger ES of studies that used wait list/treatment as usual as controls. Therefore, further double-blind investigations are warranted to elucidate the influence of the choice of comparators on the therapeutic effectiveness of EEG-NF against the symptoms of ADHD.
The present meta-analysis also shed light on the differential effectiveness of EEG-NF against the core symptoms of ADHD. Considering the lower ES of EEG-NF against hyperactivity/impulsivity than that against inattention in the present study, our findings may imply that therapeutic effects of surface EEG-NF may be more associated with improvements in inattention. Since most of our included studies used the TBR protocol, which was originally designed to enhance arousal rather than suppress impulsivity,29 its therapeutic effect may be better reflected by an improvement in arousal-related manifestations (e.g., inattention) than that in hyperactivity/impulsivity. Our results were consistent with those of previous meta-analyses7,8 that showed greater ES of EEG-NF against inattention than those against hyperactivity/impulsivity.
Another interesting finding of the present study was the effect of patients’ IQ on therapeutic outcomes. Our meta-regression analysis showed that the degree of improvement in inattention and hyperactivity/impulsivity was positively associated with a high IQ from most proximal observers. Because NF involves elements of skill acquisition and self-regulation,48,49 it is conceivable that participants with a higher IQ may benefit more from the intervention. Our finding of a positive association between IQ and therapeutic effectiveness of EEG-NF was further supported by a study showing that participants with a higher IQ could generate larger amplitudes on EEG recordings during NF training,48 indicating that IQ may affect the outcomes of NF-related learning. Therefore, a patient’s IQ may need to be taken into consideration when evaluating the possible benefits of EEG-NF.
In addition, the finding of our subgroup analysis focusing on different NF protocols showing better effectiveness of EEG-NF in studies combining 2 NF protocols (i.e., TBR + SCP) than those using only a single NF approach (i.e., TBR or SCP) suggested potential benefits of a combination approach. Nevertheless, because the result was derived from only 3 studies that adopted 2 different NF protocols29,35,42, it needs to be interpreted with caution. Finally, our demonstration of a negative association between the therapeutic effectiveness of EEG-NF against hyperactivity/impulsivity and the proportion of female participants implicated a diminished benefit of this treatment approach in the female gender regarding these symptoms. The finding may partly be explained by a generally lower severity of hyperactivity/impulsivity in females with ADHD,50 which could obscure the observed improvement in this population. Our finding implied that gender differences may need to be considered when designing EEG-NF strategies in the treatment of different targeted symptoms of ADHD.
Limitations
Although our meta-analysis included a larger number of trials (n = 21) and participants (n = 1261) than previous meta-analyses,7,8 there were several limitations. First, the lack of a double-blind design in most of our included studies rendered them susceptible to performance and detection biases. Second, we found high levels of heterogeneity across the included studies, including types of comparison groups, NF protocols, behavioural rating scales and observers of outcome measurements. Third, despite our use of subgroup analyses and meta-regression to identify potential confounding factors, some subgroup analyses included only 2–3 trials (e.g., sham/placebo controls and SCP protocols); therefore, the results need to be interpreted with caution. Fourth, our exclusion of studies using only self-rated outcome measures without incorporating objective laboratory tests into our assessment scheme means that our results could not represent treatment outcomes either from a patient’s perspective or from objective data. Nevertheless, evaluations from parents and teachers are vital in guiding appropriate support and treatment,51 given that most patients with ADHD experience symptoms at a young age. Finally, with the exception of 1 study that enrolled both adolescents and young adults (age 12–24),26 all of the other trials recruited participants younger than 18 years, possibly because of our exclusion of evaluation by self-rating scale. Therefore, the results of our meta-analysis cannot be extrapolated to the adult population.
Conclusion
Our results support the effectiveness of EEG-NF against the global symptoms as well as the symptoms of inattention and hyperactivity/impulsivity in patients with ADHD. Additionally, the positive association of IQ with improvements in inattention and hyperactivity/impulsivity encourages further studies to explore a potential effect of intellectual ability on the therapeutic outcomes of EEG-NF. Moreover, our finding of a possible benefit of a combined strategy (i.e., SCP + TBR) compared with a single NF protocol may encourage further studies to explore the advantages of combining different EEG-NF protocols in the treatment of ADHD symptoms. Finally, the inconclusive evidence linking the benefits of EEG-NF to correction of brainwave patterns through NF training and our finding of a high risk of detection and performance biases across our included trials warrant further double-blind investigations to elucidate the therapeutic effectiveness of EEG-NF against the core symptoms of ADHD.
Footnotes
↵* Contributed equally as first authors to this work.
↵† Contributed equally as corresponding authors to this work.
Contributors: W. Chung, P.-Y. Yeh, Y.-S. Cheng, H.-Y. Fen, R.-F. Tzang, C.-K. Sun and H.-J. Chiu designed the study. W. Chung, Y.-S. Cheng, C.-K. Sun and H.-J. Chiu acquired the data, which W. Chung, P.-Y. Yeh, Y.-S. Cheng, C. Liu, and H.-J. Chiu analyzed. W. Chung, P.-Y. Yeh, Y.-S. Cheng, C.-K. Sun and H.-J. Chiu wrote the article, which all authors reviewed. All authors approved the final version to be published, agree to be accountable for all aspects of the work and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
Competing interests: None declared.
Disclaimer: The contents of this article are the authors’ sole responsibility and do not necessarily represent the views of their insitutions.
- Received July 5, 2022.
- Revision received September 9, 2022.
- Accepted September 17, 2022.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/